Course Detail
Virology
List of Contents
- General Introduction
- Pathogenesis of Viral Diseases
- Antiviral Agents
- Diagnosis of Viral Infections
- Bacteriophages
- Poxviruses
- Papovaviruses
- Herpesviruses
- Adenoviruses
- Parvoviruses
- Picornaviruses
- Orthomyxoviruses
- Paramyxoviruses
- Reoviruses
- Rhabdoviruses
- Arboviruses
- Hepatitis Viruses
- Retroviruses
- Human Immudofeciency Virus
- Coronaviruses
- Other Viruses
- Prions and Prion Diseases
General Introduction
Virology is a subfield of microbiology which focuses on the study of viruses and virus-like agents. Viruses are submicroscopic, parasitic organisms of genetic material contained in a protein coat. Virology focuses on the study of virus structure, classification and evolution, reproduction, infectivity, pathogenesis and their interaction with host organism physiology and immunity, the techniques to isolate and culture them, and their use in research and therapy.
A very early form of vaccination known as variolation was developed several thousand years ago in China. It involved the application of materials from smallpox sufferers in order to immunize others. In 1717 Lady Mary Wortley Montagu observed the practice in Istanbul and attempted to popularize it in Britain, but encountered considerable resistance. In 1796 Edward Jenner developed a much safer method, using cowpox to successfully immunize a young boy against smallpox, and this practice was widely adopted. Vaccinations against other viral diseases followed, including the successful rabies vaccination by Louis Pasteur in 1886. The nature of viruses, however, was not clear to these researchers.
Viruses generally show the following features:
- They are filterable agents.
- They are obligate intracellular parasites.
- They contain a single type of nucleic acid, i.e., either DNA or RNA, but not both.
- The virion of the virus particle consists of a nucleic acid genome packaged into a protein coat (capsid), which itself is sometimes enclosed by an envelope of lipid, proteins, and carbohydrates known as envelope.
- They multiply inside the living cells by using the synthesizing machinery of the host cell.
- They replicate by the assembly of the individual components and do not replicate by division, such as binary fission.
- They have a few or no enzymes for their own metabolism. They always use host cell machinery to produce their components, such as viral messenger RNA (mRNA), protein, and identical copies of the genome.
Structure of Viruses
The viral particle that is outside the cell is called Virion. The virion may be enveloped by being surrounded by a membrane or may be nonenveloped, without being surrounded by a membrane. The virion may also contain essential or accessory enzymes or other proteins.
Clinically important viruses range in size from as small as 20nm (picornaviruses) to as large as 300nm viruses (poxvirus)
The virion consists of a nucleic acid core, the genome, surrounded by a protein coat, the capsid. The capsid with the enclosed nucleic acid is known as the nucleocapsid.
The capsid:
The nucleic acid of a virus is surrounded by a protein coat called the capsid. Each capsid is composed of a large number of protein subunits (polypeptides) called capsomeres, which form its morphological units. The structure of the viral capsid is best demonstrated by X-ray crystallography or electron microscopy.
Based on capsid structure, viruses ccan be classified into 4 types:
- Helical viruses: Helical viruses appear rod-like and may be rigid or flexible. The viral genome is found within hollow cylindrical capsid that has a helical structure. Rabies and Ebola viruses are examples of helical viruses.
- Polyhedral viruses: The polyhedral viruses appear as many-sided viruses. The viruses consist of capsids in the shape of an icosahedron. It is a regular polyhedron with 20 triangular faces. Adenoviruses have an icosahedron shape.
- Enveloped viruses: The helical and polyhedral viruses when covered by envelope are called as enveloped helical or enveloped polyhedral viruses, respectively. Influenza virus is an example of enveloped helical virus and herpes simplex virus is an example of enveloped polyhedral virus.
- Complex viruses: These include bacteriophages, which have complex structures.
The envelope
All of the negative-stranded RNA viruses are enveloped. The viruses that lack envelope are called nonenveloped or naked viruses. The virion envelope usually consists of lipids, proteins, and glycoproteins. It has a membrane structure similar to cellular membrane of the host cell. The viral envelope does not contain any cellular proteins, even though viruses are released from the host cell by an extrusion process that coats the virus with a layer of host cell plasma membrane that becomes the viral envelope. In most cases, the envelope contains proteins that are determined and encoded by viral nucleic acid. The lipid component of the envelope is usually of host cell origin.
Depending on the virus, the envelopes of the viruses may or may not be covered by spikes. The spikes are glycoprotein-like projections on the outer surface of the envelope. Most spikes act as viral attachment protein (VAP).
The structural components of envelope remain biologically active only in aqueous solutions and are readily destroyed by drying or on treatment with acids, detergents, and solvents, such as ether, leading to inactivation of virus.
Viral Nucleic Acid, Proteins, and Lipids
Viral nucleic acid
The genome of the virus consists of either DNA or RNA but never both. The DNA can be single stranded or double stranded. Depending on the virus, the DNA can be linear or circular. The RNA can be either positive sense (+) like mRNA or negative sense (-), double stranded (+/-), or ambiguous (containing + and - regions of RNA attached to it).
The total amount of nucleic acid may vary from a few thousand nucleotides to as many as 250,000 nucleotides.
Viral proteins and lipids
Viruses contain proteins, which constitute capsids. The viral protein protects the nucleic acid as well as determines the antigenic specificity of the virus. The enveloped viruses contain lipids, which are derived from the host cell membrane.
Viral Susceptibility to Chemical and Physical agents
Disinfectants
Viruses are usually more resistant to disinfectants than bacteria. Oxidizing agents such as hydrogen peroxide, potassium permanganate and hypochlorite are most effective against viruses. Water chlorination is effective at killing most viruses except hepatitis A and polioviruses.
Temperature
With only a few exceptions, most viruses are heat labile. they are inactivated within seconds at 56oC and within minutes at 37oC. Viruses, such as hepatitis B, show resistance to heating at 60oC for 60 minutes; slow viruses, such as scrapie virus, are resistant to autoclaving at 121oC for 15 minutes. The viruses are stable at low temperature. They can be stored by freezing at -35oC or -70oC. Lyophilization or freeze-drying is useful for long-term storage of the viruses. The poliovirus is an exception, as it does not withstand freeze-drying.
pH
Viruses usually remain viable in a pH range of 5-9, but are sensitive to extremes of acidity and alkalinity. Rhinoviruses are very susceptible to acidic pH, while enteroviruses are highly resistant.
Lipid solvents
Ether, chloroform, and detergents are active against enveloped viruses but are not active against nonenveloped, naked viruses.
Radiations
Viruses are readily inactivated by sunlight, ultraviolet (UV) radiations, and ionizing radiations.
Virus Replication
The genetic information for viral multiplication is present in the viral nucleic acid. Multiplication of viruses follows the basic pattern of bacteriophage multiplication, with a few differences.
The multiplication of both DNA- and RNA containing viruses, is divided into six stages:
- Attachment: also called adsorption. It is the first event in the infection of the cell by a virus. The viruses have attachment sites that attach to the complementary receptor sites on the host cell surface. These receptor proteins in the virus are distributed on surface of the virus. These receptor proteins vary from one virus to another. For example, the rabies virus binds onto the acetylcholine receptors on the neural cells.
- Penetration: Enveloped and non-enveloped viruses penetrate the cell using different mechanisms. Nonenveloped viruses enter the cell by a process called Endocytosis. In this case specifically, the process is known as viropesis. Enveloped viruses enter the cell by fusion in which the viral envelope fuses with the host's cell membrane.
- Uncoating: The process of separation of viral nucleic acid from its protein core.
- Biosynthesis: DNA viruses replicate their DNA in the nucleus of the host cell by using viral enzymes. The RNA viruses multiply in the cytoplasm of the host cell. The major differences among the multiplication process of various RNA viruses lie in how mRNA and viral RNA are produced
- Maturation: The assembly of the protein capsid is the first step in viral maturation. During maturation, the envelope protein is encoded by the viral genes and is incorporated into the plasma membrane of the infected host cell.
- Release: In case of animal viruses, the release of progeny virions usually occurs without cell lysis. The host cell is unaffected and goes on dividing to the daughter cells, which continue to release virions. Nonenveloped viruses, however, release through rupture in the host cell plasma membrane.
Viral Genetics
Viruses are obligate intracellular microorganisms. They show variation in their genomic structure by two principal methods - mutations and recombination.
Mutation
Mutation is the most important mechanism of genetic modification in viruses. Mutations occur spontaneously and readily in viral genomes causing frequent changes in the nucleic acids. This results in production of new viral strains showing properties different from parental or wild-type virus.
Mutations occuring in essential genes are known as lethal mutations.
Other mutations may produce:
Attenuated emutants - variant strains that cause less serious
infections in humans and animals.
Host range mutants - variant strains showing differences
in the tissue type and species of target cells affected by
viruses.
Plaque mutants - variant strains showing difference in
their size or their appearance in infected host cells.
Conditional mutants e.g., temperature-sensitive or cold-sensitive
mutants.
Recombination
Genetic recombination may occur when two different but related viruses infect a cell simultaneously. This leads to extramolecular genetic exchange between two viruses, leading to production of a progeny virion that possesses genes from both the viruses. There are three different types of recombination that can occur, Intramolecular recombination, Reassortment and Reactivation.
Nomenclature and Taxonomy of Viruses
A viral species is a group of virus that shares the same genetic information and ecological niche. These viral species are designated by descriptive common names, such as human herpesvirus, with subspecies, if any, designated by a number. The suffix virus is used for genus names, viridae for family names, and ales for order names. In formal usage, the family and genus names are used in the following manner: for example, family Rhabdoviridae, genus Lyssavirus, human rabies virus.
Depending on the type of nucleic acids viruses possess, they are classified into two groups: deoxyriboviruses, which contain DNA (DNA virus) and riboviruses, which contain RNA (RNA virus).
DNA Viruses
1. Adenoviridae: The members of the family Adenoviridae are medium-sized viruses measuring 20-90 nm in size. These viruses are nonenveloped, icosahedral viruses with 252 capsomeres. These viruses are mostly associated with acute respiratory diseases.
2. Poxviridae: These are large-sized, brick-shaped viruses measuring 300 X 240 X 100 µ in size. The pox (pox: pus-filled lesions) viruses are associated with skin lesions.
3. Herpesviridae: These are medium-sized icosahedral nucleocapsid viruses (100 nm) containing 162 capsomeres. They are enveloped viruses containing linear, double-stranded DNA.
4. Papovaviridae: These are small (40-55 nm) viruses containing double-stranded DNA with 72 capsomeres. They are nonenveloped viruses. They replicate in the nucleus of host cell along with host cell chromosome. This may cause host cells to proliferate, resulting in a tumor.
5. Hepadnaviridae: Hepadnaviridae (hepa: liver; dna: DNA core) are so named because they cause hepatitis and contain DNA as genome. These viruses differ from other DNA viruses by synthesizing their DNA by copying RNA using reverse transcriptase. Human hepatitis B virus, an important virus associated with human disease, is included in this family.
RNA viruses
1. Togaviridae: These viruses include arboviruses and alphaviruses. Most of these viruses multiply in arthropods as well as in vertebrates. Togaviridae (toga: envelope) are enveloped viruses containing single-stranded RNA genome. These viruses are small spherical viruses measuring 40-70 nm in size.
2. Rhabdoviridae: Rhabdoviruses (rhabdo: rod) are bullet-shaped viruses. They are enveloped, measure 130-300 X 20 nm in size, and contain a single-stranded RNA.
3. Reoviridae: They are icosahedral, nonenveloped viruses measuring 60-80 nm in size. They contain double-layered capsid enclosing 10-12 segments of double-stranded RNA. Their name is derived from the first letters of respiratory, enteric, and orphan.
4. Retroviridae: They are icosahedral, enveloped viruses measuring 100 nm in size. Many of these viruses are associated with tumors in infected hosts. One of the genera, Lentivirus includes the subspecies HIV-1 and HIV-2. Retroviruses (re: reverse, tr: transcriptase) viruses are so named because characteristically they possess the enzyme, reverse transcriptase RNA-dependent DNA polymerase.
5. Picornaviridae: Picornaviruses (pico: small) are the smallest viruses, measuring 20-30 nm in size. They are nonenveloped, icosahedral viruses with single-stranded RNA genome. These include three genera (Enterovirus, Rhinovirus, and Hepatovirus) of medical importance.
6. Orthomyxoviridae: These are medium-sized (80-120 nm) viruses. They are spherical and elongated, enveloped viruses consisting of single-stranded but segmented (eight segments) RNA genome. Influenza virus is the only virus of medical importance belonging to this group.
7. Paramyxoviridae: These are pleomorphic, enveloped viruses measuring 150 nm in size. They contain nonsegmented, single-stranded, linear RNA. Three genera have been described: Paramyxovirus, Morbillivirus, and Pneumovirus.
8. Bunyaviridae: These are enveloped, spherical viruses measuring 90-100 nm in size. The genera of medical importance include Bunyavirus, Hantavirus, Uukuvirus, Phlebovirus, and Nairovirus.
9. Arenaviridae: They are spherical, pleomorphic viruses with variable sizes (50-300 nm). They contain electron-dense, chromosome- like particles giving a sandy appearance; (arena: sand).
10. Calciviridae: These are naked nonenveloped viruses. They are small and spherical, and measure 35-39 nm in size. They show 32 cup-shaped depressions arranged in symmetry.
11. Filoviridae: They are long filamentous, enveloped viruses with variable sizes. They contain single-stranded RNA genome. The Marburg and Ebola virus are the viruses of medical importance.
Prions
Prions are infectious particles, which can transmit a disease. Prions are composed chiefly a protein without any detectable nucleic acid. Unlike conventional viruses, the prions have no virion structure or genomes and evoke no immune response in the infected host. They are extremely resistant to inactivation by heat, disinfectants, and radiation.
Viroids
Viroids are protein-free fragments of single-stranded, circular RNA that cause disease in plants. The viroids are yet to be linked to any disease in humans.
Pathogenesis of Viral Diseases
Viruses cause disease in the host first by breaking the natural protective mechanisms of the body, then evading the immune system of the host, and finally by killing off the host cells and triggering immune and inflammatory responses. Viruses replicate only inside the living cells; hence, the primary pathogenic manifestations are seen at the cellular level.
Viruses may cause disease through many defined stages including (I) entry into the body, (II) initiation of infection at a primary site (infection of the target tissue), (III) replication of virus and spread to secondary site, and (IV) clinical manifestations of the disease.
Entry into the Body
The skin is the best barrier to most viral infections. In addition to the skin, mucus membranes, ciliated epithelium, gastric acid, bile, tears, etc. confer basic natural protection against many viruses. The viruses enter the body through the respiratory tract, skin, conjunctiva, alimentary tract, and genital tract to initiate the infection by breaking these natural barriers to infection.
Respiratory tract
Many viral infections are caused by the entry of viruses through the respiratory system. The viruses enter the respiratory tract by droplets containing the viruses expelled from the nose and mouth of an infected individual through coughing, sneezing, or simply talking.
Once they enter the respiratory system, some viruses remain confined to the respiratory tract where they multiply and produce local diseases. These are known as respiratory viruses. Examples of these viruses are influenza virus, respiratory syncytial virus, rhinovirus, coronavirus, adenovirus, and Coxsackie virus A.
Other viruses enter the respiratory tract then they multiply and spread by hematogenous or lymphatic spread to other sites of the body. At these sites, the viruses replicate in large numbers and cause systemic manifestations of the disease. The examples of such viruses include measles, mumps, rubella, varicella zoster, cytomegalovirus, and Epstein-Barr virus.
Skin
Many viruses enter the skin through abrasions or breaks in the skin. Molluscum contagiosum, cowpox, vaccinia, and variola viruses enter the skin through minor lesions. Other viruses, such as papilloma virus, enter the skin through injuries on the surface of skin. Arboviruses enter the skin therough insect bites. Rabies virus enters the skin through bog bites and the bites of other animals. Hepatitis B virus and human immunodeficiency virus (HIV) enter the skin through injection.
Conjuctiva
Some viruses may enter through the conjunctiva and may cause the disease. For example, adenovirus causes local manifestations and measles virus causes systemic manifestation of the disease by entering through the conjunctiva.
Digestive System
The alimentary tract is another important route of infection for viral diseases. Viruses, such as rotaviruses, enteroviruses, adenoviruses, reoviruses, hepatitis viruses, and other gastrointestinal viruses cause clinical signs in the gastrointestinal tract. Other viruses, such as enteroviruses, adenoviruses, reoviruses, and hepatitis viruses, on the other hand, enter the body through the alimentary tract, replicate, and then they spread to other sites producing systemic manifestations of the diseases.
The digestive system has barriers to infection including (a) the acidity of the stomach, (b) the alkalinity of the small intestine, and (c) secretory enzymes found in the saliva and pancreatic secretions. In addition, intestinal mucus and secretory IgA antibodies are important and offer partial protection to the intestinal tract.
Genitalia
Some viruses can be transmitted through sexual contact and enter
the body through the genital tract.
HIV, hepatitis B virus, and hepatitis C virus are sexually
transmitted and do not produce any local lesions in the
genital tract but produce systemic manifestations.
Papilloma viruses and herpes simplex viruses (HSVs) are
also sexually transmitted but produce lesions locally in the
genital tract and neighbouring sites such as the perineum.
Congenital Transmission
A few viruses can be transmitted from an infected mother to her child in utero. These include rubella and cytomegaloviruses. Depending upon the age of the fetus, these viruses may cause malformations or even fetal death and abortion.
Initiation of Infection at Primary Site
The specificity of the virus-attachment proteins and tissue-specific expression of receptors during replication are two important properties of viruses to cause infection of target tissues.
Spread to Secondary Site
Viruses are spread in the body mainly through the circulatory and the lymphatic systems. Transport of viruses in the blood is known as viremia. After multiplication in the lymph nodes, the virus enters the blood stream, resulting in primary viremia. If the viruses are transported successfully to the liver and the spleen, replication in these organs results in production of viruses in large numbers and leads to a massive spillover of the virus into the blood stream, causing secondary viremia. This results in the onset of clinical symptoms of viral infections including the prodromal phase with high fever. Subsequently, the viruses are carried by blood stream to the target organs such as skin, brain, liver, etc. where the viruses replicate and result in the characteristic distinctive lesions specific for the disease.
Clinical Manifestations of Viral Diseases
Clinical manifestations of viral diseases depend on the interaction between the virus and host factors. The outcome of the infection depends on the Age, general health, and immune status of the host, the infective dose of the virus, and the genetics of the host and the virus. The immune status of the host is a related factor since this may be induced by chemical agents or other diseases, rather than based on genetics alone.
Pathogenesis at the Cellular Level
Cells can be classified into three types based on the interaction they have with the virus.
- A permissive cell is a cell that allows replication of a particular type of strain of virus by providing biosynthesis compounds to enable the virus to replicate.
- A nonpermissive cell does not provide any biosynthesis compound, hence does not support replication of the viruses.
- A semipermissive cell may support some but not all the stages in viral infection.
Replication of virus in cell may cause a broad spectrum of effects, ranging from nonapparent cellular damage to rapid cell destruction.
- In some cases, viruses infect cells and replicate within the cells without causing any cellular injury to the host cells. This is known as steady state infection.
- In cell cultures, viruses produce demonstrable cellular changes in the infected cells known as cytopathic effects (CPEs). Cytopathic effect, or CPE, is a term that denotes any visible change in the appearance of target cells infected by viruses.
- Some viruses may cause cell death or even cell lysis. For example, polioviruses cause death of cells (cytocidal effect) and even lysis of the cells (cytolysis). HSV, poliovirus, togaviruses, and poxviruses cause cell death by inhibition of cellular protein synthesis. These viruses produce proteins that inhibit synthesis of cellular DNA and/or RNA. These viruses also produce other proteins, which break down host cellular DNA to components that serve as substrates for replication of the viral genome.
- Molluscum contagiosum causes proliferation of cells, and oncogenic viruses cause malignant transformation of cells.
- Some other viruses may cause alterations in the cells' morphology, functional properties or antigenicity.
Immunity in Viral Infections
Humoral immunity
Circulating IgG and IgM antibodies are effective against viruses in blood and tissue spaces while IgA plays an important role against viruses replicating on the mucosal surfaces. The antibodies against surface antigens are usually more effective than against internal antigens. The antibodies produced against surface antigens also vary in their ability to reduce infectivity of viruses. For example, the antihemagglutinin antibodies produced against hemagglutinin antigen of influenza virus neutralizes infectivity of the influenza virus, while antineuraminidase antibodies against neuraminidase antigen are not effective in neutralizing the infectivity of the virus. Humoral antibodies, although are protective, in some instances may cause injuries to host cells and contribute to pathogenesis of disease.
Cell-mediated immunity
CMI is essential for lysis of target cells in the infections caused by enveloped viruses and also in noncytolytic infections caused by hepatitis A virus. CMI also plays a major role in recovery from viral infections. An individual deficient in CMI results in failure to resolve the infection, which may lead to persistent viral infection, chronic disease, and even death of the patient. CMI also contributes to pathogenesis of diseases by inducing T-cell-induced inflammatory and hypersensitivity reactions. For example, the typical clinical manifestation of measles and mumps are due to CMI induced inflammatory response rather than the cytopathologic effect of virus itself. Infection by some viruses is usually associated with suppressed host immunity; for example, infection by measles virus causes a temporary depression of delayed hypersensitivity to tuberculin antigen and infection by HIV causes a depressed CMI following depletion of CD4+ helper T cells.
Other factors that influence the development of infection include:
- Age: Age is the most important factor in determining the susceptibility of the host to viral infections. Most viral infections are common and are more serious in the persons at extremes of age, such as infants, children, and elderly population.
- Poor nutrition: Poor nutrition or malnutrition affects the immune system of the infected person because tissue- regenerating capacity is usually decreased in a person with malnutrition.
- Genetic make up: Genetic make up of a person also plays an important role in determining the outcome of viral infection. The genetic differences might affect immunity genes and or the ability of the host to prevent viruses from entering the body. Genetic differences may also influence the severity of disease once the virus is introduced into the body.
Epidemiology of Viral Infections
A viral infection in a community may occur as an (a) outbreak, (b) epidemic, or (c) as pandemic:
- An outbreak of a viral disease usually occurs from a common source, for example, infected food, and is seen only in clusters of people.
- Epidemics occur in a large geographical area. An epidemic usually occurs due to introduction of a new strain of virus into an immunologically susceptible population, e.g., influenza epidemic.
- Pandemics are usually worldwide epidemics resulting from the introduction of a new virus, e.g., HIV. Pandemics of influenza have occurred as a result of introduction of new strains of influenza viruses. The SARS-CoV-2 (Covid19) can also be described as a pandemic due to it being a new strain of coronaviruses and its global distribution.
Prevention of Viral Diseases
Viral diseases can be prevented by:
- Adopting good hygiene
- Control of the vectors
- immunization of the population. Immunization of the population by vaccines is the best means for the control of many diseases. Vaccines are available against many viral diseases (poliomyelitis, rabies). These protect the population against infection by viruses.
Antiviral Agents
Unlike most bacteria, viruses are obligate intracellular pathogens that use biosynthesis mechanisms and enzymes of the host cells for replication. The first antiviral drugs to be used were like selective poisons that targeted cells with intensive DNA and RNA synthesis. Recently used antiviral drugs, however, act specifically against virus-coded enzymes or structures of the virus that are important for replication of the viruses. Marboran was the first antiviral drug used clinically for effective treatment of poxvirus infection in 1960. The compound was used successfully against eczema vaccinatum and smallpox. Subsequently in 1962, an antineoplastic agent idoxuridine was found to be effective for treatment of herpetic eye infection, and amantadine (a molecule with an unusual structure) was found effective for treatment of influenza A virus. In 1970s, acyclovir (ACV) was used most successfully for treatment of herpes virus infection by administering the drug parenterally.
Mechanism of Action of Antiviral Drugs
Viral infections may be inhibited at multiple levels include:
- Attachment
- Penetration and uncoating
- Transcription of viral nucleic acid
- Translation of viral mRNA
- Protein synthesis
- Replication of viral DNA
- Nucleoside biosynthesis and nucleoside scavenging
- Assembly and release of viral progeny
Classification of Antiviral Drugs
Nucleoside Analogs
Nucleoside analogs that act by inhibiting the enzyme viral polymerase are generally activated by phosphorylation by cellular or viral kinases.
The commonly used nucleoside analogs are acyclovir, valacyclovir, penciclovir, and famciclovir, ganciclovir, azidothymidine (AZT), ribavirin, and dideoxynucleosides (dideoxyinosine, dideoxycytidine, stavudine, and lamivudine).
- Acyclovir: (ACV) is a synthetic guanine nucleoside analog. It differs from the nucleoside guanosine by having an acyclic (hydroxyethoxymethyl) chain instead of a ribose or deoxyribose sugar. The ACV has selective action against herpes viruses, such as herpes simplex virus (HSV) and varicella zoster virus.
- alacyclovir: Valacyclovir is the valyl ester derivative of ACV that is well absorbed. Its bioavailability is 2-5 times more than ACV and is usually recommended for the treatment and suppression of genital herpes infection.
- Penciclovir: Penciclovir is a guanosine analog. It has a higher affinity for HSV thymidine kinase than ACV but has a lower affinity for HSV DNA polymerase than ACV. It acts by inhibiting viral DNA polymerase and also as a chain terminator. It is used for the treatment of genital herpes infection.
- Ganciclovir: It differs from ACV in having a single hydroxymethyl group in the acyclic side chain. It acts as a chain terminator in subsequent termination of viral DNA replication. It is highly effective against all herpes viruses including cytomegalovirus. It is more active against cytomegalovirus (CMV) than ACV.
- Azidothymidine: Azidothymidine (AZT) is the synthetic analog of thymidine and was the first useful antiviral agent to be reported for treatment of HIV infection. It acts by blocking the synthesis of proviral DNA by inhibiting viral reverse transcriptase. The latter is 100 times more susceptible to inhibition by AZT than host cellular DNA polymerase.
- Ribavirin: Ribavirin is a synthetic analog of the nucleoside guanosine. Ribavirin triphosphate is the active form of the drug. It differs from guanosine by having a base ring, which is incomplete and is open. Ribavirin is effective against many DNA and RNA viruses. It acts mainly by preventing replication of the viruses by inhibiting nucleoside biosynthesis, mRNA capping, and other processes essential for viral replication.
- Dideoxynucleosides: Dideoxynucleosides (e.g., dideoxyinosine, dideoxycytidine, stavudine, and lamivudine), the analogs of nucleosides, have been synthesized for use against HIV. These agents act by inhibiting HIV replication by blocking the enzyme reverse transcriptase. These compounds are usually recommended for the treatment of AIDS in patients not responding to therapy with AZT. These are also used in combination with AZT for treatment of the AIDS cases.
- Other Nucleoside Analogs: These consist of a number of compounds including:
- Idoxuridine
- Trifluorothymidine
- Fluorouracils
- Adenine arabinoside
Non-Nucleoside Polymerase Inhibitors
Non-nucleoside polymerase inhibitors include foscarnet and related phosphonoacetic acid. These inhibitors inhibit replication of viruses by binding to the pyrophosphate binding site of the DNA polymerase to block binding of nucleotides.
Foscarnet specifically inhibits DNA polymerase of all herpes viruses and reverse transcriptase of the HIV. The compound has also shown antiviral activity against hepatitis B virus. Nevirapine, delavirdine, and efavirenz are the other nonnucleoside polymerase inhibitors with different mechanisms of action.
These compounds are usually given in combination with other nucleoside analogs to delay or prevent emergence of drug resistance in HIV.
Protease Inhibitors
Saquinavir, indinavir, ritonavir, nelfinavir, and amprenavir are some of the examples of protease inhibitors. These agents act specifically on the unique structure of HIV protease, which is essential for the production of a functional HIV. Human immunodeficiency virus strains showing resistance to these drugs occur through mutation of the HIV protease. Hence, a combination of protease inhibitor with AZT and a nucleoside analog is usually recommended to reduce replication of viruses to minimum undetectable levels.
Interferon
There are three classes of interferon: (i) interferon alpha (IFN-α), (ii) interferon beta (IFN-β), and (iii) interferon gamma (IFN-γ). IFN-α occurs as at least 15 subtypes, the genes for which show 85% homology. Interferons are produced by leukocytes and many other cells in response to infection by virus, double-stranded RNA (dsRNA), endotoxin, and mutagenic and antigenic stimuli.
Interferons exert antiviral
effect by several pathways as follows:
1. They cause increased expression of class I and class II MHC
(major histocompatibility complex) glycoproteins, thereby
facilitating the recognition of viral antigens by immune
system.
2. They activate the cells, such as natural killer cells and
macrophages, the cells with the ability to destroy virusinfected
targets.
3. They directly inhibit replication of viruses.
Interferons are now being increasingly used for treatment
of chronic hepatitis B and C virus carriers who are at risk
to progress to cirrhosis and hepatocellular carcinoma.
Other Antiviral Drugs
Amantadine (Adamantanamine hydrochloride, symmetrel) and rimantadine are anti-influenza drugs useful for treatment of influenza virus infections. These are not effective for treatment of influenza B or C viruses. These act specifically against influenza A virus by their ability to bind and to block protein channel by the matrix protein (M2) of the influenza A virus.
Zanamivir (Relenza) and oseltamivir (Tamiflu) are the antiviral compounds with clinical efficacy against both the influenza A and B viruses. They are potent inhibitors of the influenza neuraminidase.
Laboratory Diagnosis of Viral Diseases
The clinical manifestations of most viral diseases may be nonspecific. However, they provide a clue in the diagnosis of viral infections by exclusion of common causes by bacteria, parasites or fungi etc. Laboratory diagnosis, therefore, plays an important role in confirming viral etiology of already suspected viral diseases.
Laboratory procedures for diagnosis of viral infections
| Approaches | Method |
|---|---|
| Demonstration of virus-induced cytopathic effects (CPEs) in the cells | Characteristic CPEs include (a) change in cell morphology, such as rounding of cells, or rounding and aggregation of cells, (b) syncytia formation, and (c) inclusion bodies formation |
| Direct detection of viruses | Electron microscopy, fluorescence microscopy, and light microscopy |
| Virus isolation | Animal inoculation, embryonated egg inoculation, and cell culture |
| Detection of viral proteins and other enzymes | The enzyme-linked immunosorbent assay (ELISA), direct immunofluorescence assay, RIA, etc. |
| Detection of viral genome | DNA probes, dot blot or Southern blot analysis, Northern blot or RNA:DNA probe hybridization, polymerase chain reaction (PCR), and reverse transcriptase PCR (RT PCR) |
| Viral serology | Hemagglutination inhibition (HI) test, neutralization test (NT), indirect fluorescent antibody (IFA) test, ELISA, RIA, latex agglutination test (LAT), and Western blot |
Laboratory specimens for diagnosis of viral infections
| Body System | Direct examination | Isolation | Serology |
|---|---|---|---|
| Respiratory system | Nasopharyngeal aspirate (IF, EM) | Throat swab, throat washings | Paired sera |
| CNS | Brain biopsy (IF, EM), CSF (EM, IF) | Feces, blood (for arbovirus), CSF, and brain biopsy | Paired sera |
| Skin | Vesicular/pustular fluid (EM, ID), ulcer scrapings (EM), and crusts (EM, ID) | Macular/papular scrapings, vesicular/pustular fluid, ulcer scrapings, crust, urine | Paired sera |
| Eye | Conjunctival scrapings and smears (LM, IF) | Conjunctival scrapings or swabs | Paired sera |
| Liver | Serum and feces | Blood (for yellow fever) | Serum |
| Congenital infections | Nil | Throat swab, products of conception | Single sera (mother and baby) |
| Gastro-intestinal tract | Stool (antigen detection, EM for rotavirus) | Not cultured | Paired sera (ELISA) |
| Key: IF, immunofluorescence; EM, electron microscope; ID, immunodiffusion; LM, light microscopy. |
Bacteriophages
Bacteriophages (or phages in short, are bacterial viruses i.e., they are obligate intracellular parasites that multiply inside bacteria by making use of some or all of the host biosynthesis machinery.
The activities of bacteriophage were first described by Twort in 1915, who described it as an infectious agent that distorted the appearance of the colonies of staphylococci. Subsequently, d'Herelle in 1979 demonstrated the lytic activities of the culture filtrate on bacterial colonies. He suggested that the lytic agent was a virus and gave it the name bacteriophage. remember the term phage means eat, therefore bacteriophage are viruses that 'eat' bacteria.
Structure of Bacteriophages
Most of the phages consist of single, linear, and doublestranded DNA genome. This genome is surrounded by a protein coat known as phage capsid. Large phages usually consist of a head and a tail. The head encloses the genome, and the tail is used as an organ of attachment as well as the conduit Bacteriophages through which phage DNA passes into the host cell. The best studied phages are the T-even phages (T2, T4, T6, etc.) that infect the bacteria Escherichia coli. These phages are traditionally considered as the prototype for describing the morphology of bacteriophages.
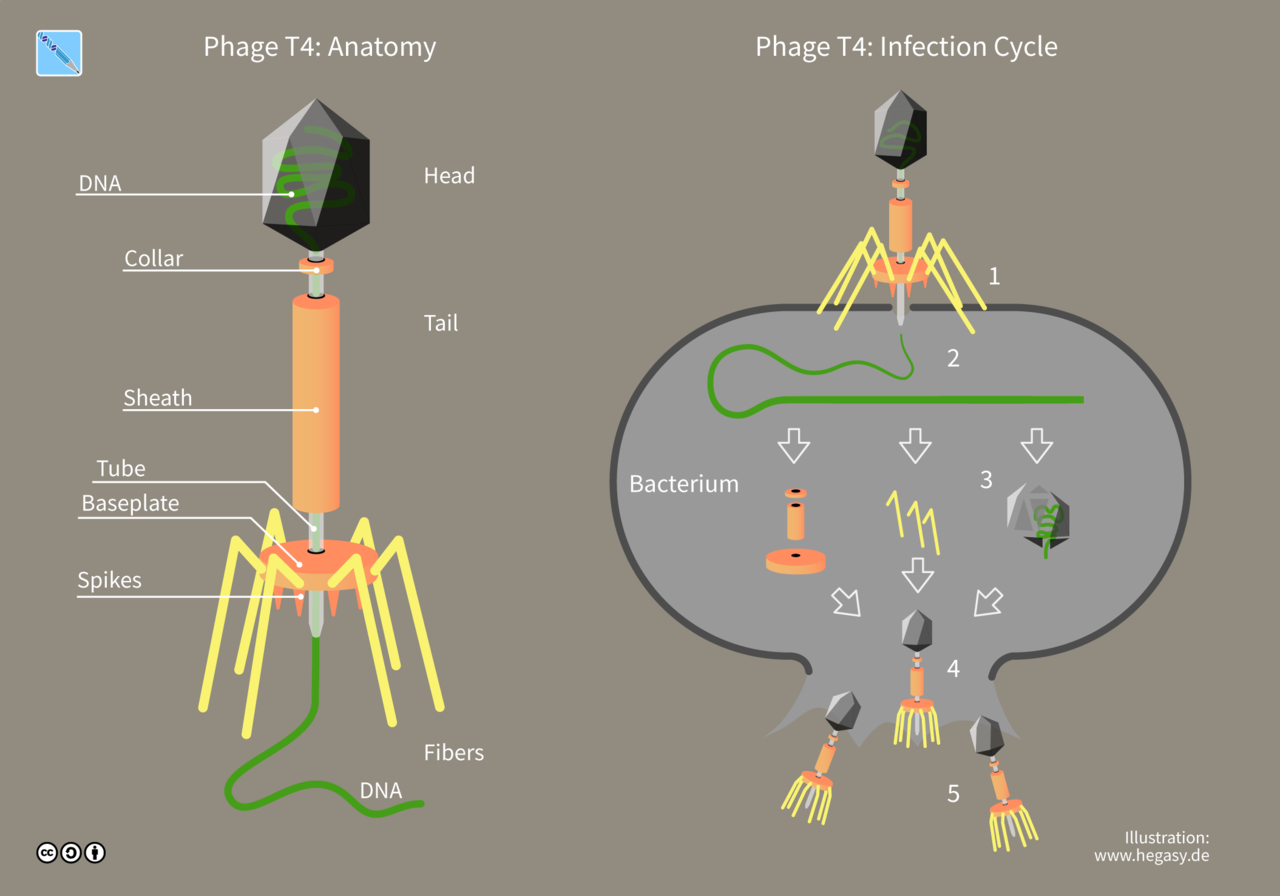
Structure of bacteriophages and an illustration of how they infect and multiply in bacterial cells
Uses of Bacteriophages
Bactriophages can be used for the following purposes:
- As a model for study of virus host interaction
- They play an important role in transmission of genetic information by process of transduction.
- Used as cloning vectors for genetic manipulations.
- Phage typing
Bacteriophage Typing
When a suspension of phages is deposited on the lawn culture of a susceptible bacterium, an area of clearing occurs after incubation due to lysis of the susceptible bacteria by the phages. These zones of lysis are called plaques. The shape, size, and nature of plaques are characteristic for different phages. Since a single phage particle is capable of producing one plaque, plaque assay can be used for titrating the number of viable phages in preparation. On the basis of this phenomenon, many bacterial species can be divided into various phage types. Phage typing has been used in epidemiological study of infections or outbreaks caused by Staphylococcus aureus, Salmonella spp., Vibrio cholerae, and many other bacteria.
Poxviruses
The family Poxviridae include a large group of DNA viruses that are morphologically similar and share a common nucleocapsid protein. Poxviruses are the largest and most complex viruses that occur in humans, birds, animals, and insects.
There are eight genera in this family, of which at least four genera cause diseases in humans. These include:
- Orthopoxvirus: The genus Orthopoxvirus includes the poxviruses of mammals, such as smallpox (variola), vaccinia, monkeypox, cowpox, buffalopox, rabbitpox, mousepox, and camelpox viruses.
- Parapoxviruses: These include the viruses of ungulates that may cause occasional infections in humans. These viruses are orf viruses, pseudocowpox virus, deerpox viruses, and bovine papular stomatitis virus.
- Yatapoxviruses: These include tanapox viruses and yabapox viruses that are found mainly in Africa.
Molluscipoxviruses: These include molluscum contagiosum virus.
Variola (Smallpox) Virus
Morphology
- The variola virus is a large, brick-shaped virus measuring 300 * 200 * 200 nm, almost visible by light microscopy
- The virion consists of protein (90%), lipid (5%), and DNA (3%).
- The viral genome consists of a large, double-stranded, linear DNA that is fused at both ends.
- The extracellular virion possesses two envelopes, while the intracellular virus has only one envelope.
Viral replication
Poxvirus replication is complex. Among the DNA viruses, they are unique in that the complete replication cycle of the virus occurs in the cytoplasm of the host cell. The virus encodes the enzymes required for mRNA and DNA synthesis essential for genetic replication.
Susceptibility to physical and chemical reagents
Variola virus is most stable at low temperature and low humidity. It remains viable for months at room temperature, if protected from sunlight and in the cold or when freeze-dried for years. It is resistant to action of 50% glycerol and 1% phenol. Even though enveloped, the virus is not susceptible to ether, hence is not inactivated by ether. It is susceptible to ultraviolet light and other irradiations and is also readily inactivated by formalin and oxidizing disinfectants.
Virus Isolation and Animal Susceptibility
Chick embryo
Both variola and vaccinia viruses grow in the chick embryos. They produce pocks on chorioallantoic membrane (CAM) of 11-13 days’ old chick embryo within 48-72 hours.
Laboratory animals
Variola virus causes experimental infection only in monkeys. Intranasal infection of monkeys by variola virus causes smallpox in monkeys with generalized skin lesions.
Pathogenesis and Immunity
Smallpox caused by variola virus is acquired by respiratory route through inhalation of nasal, oral, or pharyngeal droplets. The infection can also be acquired by direct contact with infected skin or fomites.
The virus replicates at the site of inoculation and spreads to lymph nodes draining the site of mucosal entry. The virus replicates in the lymphoid tissues, causes transient viremia, and infection of the reticuloendothelial cells. This is followed by a secondary phase of multiplication in these cells, leading to a secondary viremia.
The viruses then enter the skin, localize in the blood vessels of the skin, and produce characteristic rash of the smallpox. The rash is due to the replication of virus in the skin.
Variola infection is characterized by development of both humoral and cellular immunities. Humoral immune response includes the appearance of hemagglutination inhibition (HI), complement fixing (CF), and neutralizing (NT) antibodies within first to third week of infection. Humoral antibodies are not protective. Cell-mediated immunity plays an important role in controlling and resolving the disease. Virus-specific T cells control the spread of viruses by causing lysis of infected cells in the reticuloendothelial cells in the skin.
An attack of smallpox gives complete protection against reinfection. Vaccination confers immunity, which lasts about 10 years.
Smallpox was a highly infectious disease. Respiratory secretions and exudates of the skin lesions were the most common sources of infection.
Clinical Syndrome
The incubation period varied from 10 to 14 days. The prodromal phase, which correlated with the phase of viremia, was the first to appear.
The smallpox rash was characterized by skin lesions that are in the same stage of evolution, unlike those lesions seen in chickenpox. The lesions in chickenpox appear in successive waves and in various stages, such as vesicles, pustules, and scabs. The skin lesions first appear on the face and extremities and then spread centrifugally to the trunk.
Overwhelming toxemia was the usual cause of death in patients with smallpox. There were two variants of smallpox: variola major and variola minor. The variola major was associated with a fatality rate of 25-30%, while variola minor was associated with a low fatality rate of less than 1%.
Epidemiology
The last case of naturally occurring smallpox was detected in Somalia in 1977. The last recorded case in humans, which was due to an accidental laboratory infection, was reported in England in 1978. The WHO in 1980 declared that smallpox was eradicated from the world. At present, the only remaining known virus isolates are stocked in the laboratories at the Center for Disease Control and Prevention (CDC) in the United States and at the Vektor Institute in Russia.
In the 1990s, it was learned that the smallpox virus has been used by some countries in their biological warfare program. It is not known how many countries still possess the virus in their laboratories.
Diagnosis
Smallpox was usually diagnosed clinically. The main criteria
for clinical diagnosis of smallpox included:
1. Febrile prodromal phase occurring 1-4 days before the onset
of rashes;
2. Characteristic smallpox lesions of the skin (deep, firm,
round), which could be umbilicated or confluent; and
3. Skin lesions in same stage of development on any part of the
body.
Isolation of the virus
Isolation of the virus in the laboratory is carried out by inoculation in the chick embryo and in cell culture. This is necessary for rapid and accurate identification of poxvirus infections.
Serodiagnosis
Serological tests were useful to confirm the diagnosis of poxvirus infection. Indirect immunofluorescent antibody test and HI, CF, and NT tests are available for demonstration of serum antibodies that appear after first week of infection.
Treatment
No specific antiviral agents are available against variola virus. Methisazone is of some value against some poxviruses. It is recommended only for chemoprophylaxis but not for treatment. Vaccinia immune globulin is recommended for treatment of all complications except postvaccinated encephalitis.
Prevention and Control
Smallpox was the first disease to be eradicated by successful immunization program.
Vaccinia Virus
Vaccinia virus is unique in that it is an artificial virus and does not occur in nature as such. Vaccinia virus was used as the smallpox vaccine. than variola, as it is safer to work with. The virus is also being used as a vector for development of recombinant vaccines. Both vaccinia and variola viruses show similarity in their morphology. They, however, can be differentiated by their growth properties and host range.
Vaccinia causes infection by accidental inoculation of the skin. At the site of inoculation, the lesion begins as maculopapular rash, progressing to vesicles, pustules, and finally leading to formation of scab. The lesion heals with formation of marked scarring. Vaccinia virus in patients with eczema causes eczema vaccinata.
Monkeypox
disease similar to smallpox. This virus was first isolated in 1958 from captive monkeys in Copenhagen. First human infection with this virus was described in the early 1970s. Cases of monkeypox have been described as a rare zoonotic infection in West and Central Africa, especially in Zaire. Clinically, monkeypox cannot be distinguished from smallpox. The condition manifests with development of a generalized pustule, rash, fever, and toxemia. Electron microscopy is useful to demonstrate the virion particles in clinical specimens for diagnosis of the condition.
Buffalopox
Buffalopox, believed to be caused by vaccinia virus, occurs among buffaloes in India. In the infected buffaloes, the viruses produce pustular lesions on the teats and udders of lactating buffaloes. The individuals, such as milkmen, coming in contact with these infected buffaloes suffer from the infection. The lesions usually develop on the hands and faces of the milkmen.
Cowpox
Cowpox infection in cows produces ulcers on the teats and udders. It may spread to other cows and humans during the process of milking. Natural cowpox infection has also been noted in wild animals kept in zoos including cheetahs and elephants and also in domestic cats. Milker's node or paravaccinia is an occupational disease acquired by humans during the process of milking of infected cows. The common lesions include small ulcerating nodules
Orf
Orf is an infection of humans caused by virus of contagious pustular dermatitis of sheep and goats. The lesions usually present on hands, forearm, or occasionally on the face. The lesions heal without forming any scars. The virus resembles paravaccinia virus morphologically.
Molluscum Contagiosum
Molluscum contagiosum is a poxvirus unique to humans. The virus is spread by close contact, often through sexual contact. The virus causes a disease of the skin usually seen in children and young adults. It causes small, pink, papular pearl-like benign tumors of the skin or mucous membranes. Lesions are more commonly found on the trunk and anogenital areas.
Molluscum contagiosum in patients with HIV may cause chronic and extensive skin lesions. Sections of the nodular lesions show hyaline acidophilic inclusion bodies called molluscum bodies. Their bodies are large and measure 20-30 µm in size and are composed of large numbers of virion particles embedded in a protein matrix. Humans are the only susceptible host. The virus cannot be grown in embryonated eggs, tissue cultures, or animals.
Tanapox
Tanapox virus was first isolated from the cases of febrile illness that occurred along the Tana river in Kenya during 1950s. The virus produces single, pock-like vesicular lesion on the skin, which usually do not progress to form pustules.
Yabapox
Yabapox virus produces large benign tumors in monkeys. It causes benign histiocytomas 5-20 days after muscular or subcutaneous inoculation to monkeys. Such lesions have been reported in persons handling affected monkeys.
Papovaviruses
The term papova is derived from the first two letters of the names of the viruses (pa, papillomaviruses; po, polyomaviruses; and va, vacuolating agents). The family is divided into two genera Papillomavirus and Polyomavirus. The papovaviruses encode proteins that promote cell growth. They induce both lytic infections and tumors, which may be benign or malignant.
Human Papillomaviruses
Human papillomaviruses (HPVs) are the causative agents of papillomas, which are the benign tumors of squamous cells or warts on the skin. These are also associated with cancerous conditions in humans, such as cervical carcinoma.
Morphology
Human papillomaviruses show the following features:
- They are double-stranded DNA virus and non-enveloped.
- They are slightly larger than polyomaviruses and measure 50-55 nm diameter.
- They have an icosahedral capsid, composed of 72 capsomeres.
- The viral genome is a supercoiled, double-stranded DNA composed of approximately 3000 base pairs (bp). The DNA encodes seven to eight early genes (E1-E8) and two late (L1 and L2) genomes.
Viral Replication
Papillomaviruses show affinity for epithelial cells of the skin and mucous membranes. The viruses are dependent on the specific factors that are present in sequential differentiated states of epithelial cells.
The early genes of the virus are responsible for growth of cells and facilitate replication of viral genome during cell division. The virus-induced cell growth causes thickening of the basal and prickle cell layer of stratum spinosum.
Pathogenesis and Immunity
Human papilloma viruses show high degree of host specificity. They show a high predilection for the skin and also for mucous membrane. HPV infection is acquired by close contact.
After infection, the virus replicates in the squamous epithelium of the skin to cause warts and in the mucous membrane to induce epithelial proliferation, such as oral, genital, and conjunctival papillomas. The presence of the koilocytes is the hallmark of the HPV infections of the skin. These koilocytes are the enlarged keratocytes with well-demarcated halos surrounding small nuclei. The HPV infection always causes a localized infection and the warts usually resolve spontaneously possibly as a result of immune response.
The exact mechanism responsible for resolution of papillomas is not known. Cell-mediated immunity, however, appears to play an important role in the resolution of the disease.
Clinical Syndromes
Human papilloma virus infection causes: (a) cutaneous warts, (b) benign head and neck tumors, (c) genital warts, and (d) cancerous conditions in humans
Cutaneous warts
Cutaneous warts are commonly caused by HPV-2, HPV-4, and HPV-7. Warts usually develop on the hands and feet after incubation period of 3–4 months depending on the HPV type and the site of infection. They may appear flat, plantar, or dome shaped. The plantar and flat warts are most common in children and young adults. Cutaneous warts are usually benign and self-limiting.
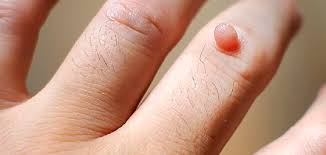
Cutaneous wart
Benign tumors of head and neck
These include oral papilloma, laryngeal papilloma, and
conjunctival papilloma.
Oral papillomas are usually single but may be multiple.
They are sessile, verrucous, and white with raised borders.
These lesions usually appear on the lips, hard palate, or gingiva.
Focal epithelial hyperplasia or Heck disease is commonly caused
by HPV-13 and HPV-32.
Laryngeal papillomas are life-threatening conditions in children, caused by HPV-6 and HPV-11. This is the most common
benign epithelial tumor of the larynx.
Genital warts
Genital warts or condyloma acuminata is caused by HPV-6 and HPV-11. The condition typically manifests as solitary or multiple cerebriform and pink lesions, which appear more commonly on the nonkeratinized mucosa than on the keratinized mucosa.
Cancerous conditions
Certain types of HPV-commonly, HPV-16 and less frequently, HPV-18, HPV-33, HPV-35-have been associated with oral premalignancy and malignancies in humans. These conditions are associated with verruciform proliferations in the oral cavity. Oral premalignant lesions and oral squamous cell carcinoma caused by HPV-16 and HPV-18 are most commonly associated with intraepithelial cervical neoplasia and cancer. The conditions progresses from mild to moderate neoplasia to severe dysplasia or carcinoma in situ during a period of 1-4 years.
Epidemiology
HPV infections are found worldwide. Human papilloma viruses have been detected in the oral cavity of estimated 6-10% of children and adolescents, and in 5-80% in healthy adults. HPVs are found in genital secretions and skin sheddings, which contain virus. Infected humans are the main source of infection. Asymptomatic shedding of viruses in body secretions facilitates transmission of infection to other human hosts.
The infections are transmitted primarily by skin-to-skin contact and by genital contact. Type 6 and 11 may be transmitted by passage through infected birth canal as for laryngeal papilloma.
Laboratory Diagnosis
The diagnosis of wart is made by histopathological examination. Demonstration of hyperplasia of the prickle cells and excessive production of keratin (hyperkeratosis) is diagnostic of the condition. Human papilloma virus infection can be detected by the demonstration of round coalesced cytotic squamous epithelial cells occurring in clumps in Papanicolaou smears.
Cell cultures are not useful, because HPVs do not grow in cell lines. Serology is rarely used. The typing of virus isolates may be carried out by immunohistochemical detection of HPV structural proteins.
Treatment
Warts are regressed during the course of time over a period of months to years. The warts can be removed by surgical cryotherapy, electrocautery, or chemical reagents.
Prevention and Control
No specific preventive measures are available against HPV infection. Avoidance of direct contact with infected warts may prevent transmission. Safe sexual practice will be useful to prevent sexual transmission of HPV.
Polyomaviruses
Polyomaviruses (poly, many; oma, tumor) are smaller viruses than papillomaviruses, measuring 45 nm in diameter. They are nonenveloped viruses with a 72-capsomere icosahedral capsid. Viral genome is a double-stranded DNA containing less nucleic acid, approximately 5000 bp.
Different polyomaviruses show different host specificities. The pathogenesis of the human polyomavirus infection in humans depends on the immune status of the host. In the immunocompetent host, the replication of viruses is inhibited. Suppression of immunity in patients receiving organ transplantation or suffering from AIDS results in reactivation of latent JC and BK viruses. In these patients, reactivation of viruses leads to shedding of viruses and symptomatic infection. All the polyomaviruses (BK, JC, and SV40) are known to cause tumor in animals, such as hamsters. These viruses, however, are not associated with any tumors in humans.
Electron microscopy is useful to detect JC virus in brain tissue from the cases of PML and from urine of kidney transplant recipients. Immunoperoxidase and in situ immunofluorescence are rapid detection methods for detection of viral antigen in brain tissue obtained by biopsy or at autopsy. BK polyomavirus is isolated from urine by culture in human diploid fibroblasts; JC virus is isolated from urine and brain tissue by culture in human fetal glial cell culture. Hemagglutination inhibition test is performed to differentiate these two viruses.
Herpesviruses
The herpesviruses are large, enveloped DNA viruses. They exhibit many common features, such as similar morphology of virions, basic mode of replication in the host cells, and capability to establish latent and recurrent infections.
The DNA core consists of a linear double-stranded DNA (dsDNA) molecule with molecular weight varying from 125 to 229 kilobase pairs (kbp). The core is surrounded by an icosahedral capsid containing 162 capsomeres.
Envelope is the outermost component and is composed of lipids. It is derived from the modified host cell nuclear membrane through which the naked virions project during replication. It carries surface spikes about 8 mm long.
Herpesviruses replicate in the host cell nucleus, and both replication and assembly occur in the nucleus. The herpesvirus encodes for several glycoproteins that facilitate viral attachment, fusion, and immune evasion. The virus buds from nuclear membrane and is released by exocytosis and cell lysis.
All human herpesviruses are included in the family Herpesviridae, which is divided into three subfamilies based on viral characteristics, pathogenesis of the disease, and clinical manifestation of the disease.
Herpes Simplex Virus
Herpes simplex viruses (HSVs) are extremely host-adapted viruses that can cause a wide variety of illness in infected human hosts. There are two types of the HSVs: (a) herpes simplex virus type 1 (HSV-1) and (b) herpes simplex virus type 2 (HSV-2). HSV-1 is transmitted primarily by contact with infected saliva, whereas HSV-2 is transmitted by sexual contact or by genital tract infection to newborn from an infected mother.
Morphology
HSVs, like other herpesviruses, are large, enveloped, icosadeltahedral viruses. Both HSV-1 and HSV-2 are structurally and morphologically similar. The virus contains a dsDNA. The genomes of both HSV-1 and HSV-2 are similar in organization and show a higher degree of sequence homology.
The unique feature of the DNA genome is that it encodes for as few as 80 polypeptides. Half of the proteins are required for replication of viruses, whereas other proteins help in interaction of the viruses with different host cells and immune response
Viral replication
Herpes simplex virus (HSV) grows very rapidly in infected cells, requiring only 8-16 hours for completion. The virus infects most types of cells in human hosts and usually causes lytic infections of the fibroblasts and epithelial cells. After entry into the cell, the virion is uncoated, genome is released, and the genome DNA enters into the nucleus. The virion acquires its envelope by budding through the nuclear membrane.
Like other enveloped viruses, HSVs are sensitive to treatment with acid, fat solvents, detergents, and drying. They are readily inactivated in the conditions prevalent in the gastrointestinal tract.
Viral Isolation
Chick embryo: The virus grows on the chorioallantoic membrane of the embryonated egg and produces small, white, shining, nonnecrotic pocks measuring less than 0.5 mm in diameter.
Culture: Herpes simplex virus grows in a variety of primary and continuous cell lines. The viruses grow readily on HeLa cells, Hep-2 cells, human embryonic fibroblasts, and rabbit kidney cells.
Pathogenesis and Immunity
Herpes simplex virus infection is initiated by direct contact and depends on the infected tissues whether oral, genital, or brain, etc. The infection occurs by inoculation of virus into susceptible mucosal surfaces, such as the oropharynx, conjunctiva, or cervix or through small abrasions on the skin.
Viruses replicate at the site of entry in the skin or mucous membrane. The neuroinvasiveness (the ability of virus to invade the brain), neurotoxicity (ability to multiply in the brain and destroy the brain), and its latency (ability to remain in a nonreplicating stage in the dorsal root ganglia of the central nervous system, or CNS) are the properties of HSV that influence the course of infection in an infected host. The virus replicates in the infected cells at the base of the lesion and infects the innervating neuron. The virus then returns back to the initial site of infection and may cause inapparent infection or produce vesicular lesions Thin walled vesicles, which break down, leaving tiny superficial ulcers are the typical lesions caused by HSV. These vesicles heal without forming any scars. The vesicle fluid contains infectious virions.
Various stimuli, such as physical or emotional stress, trauma, fever, and sunlight can induce a recurrence in which the virus travels back down the nerve, leaving lesions to develop at the skin, at the same spot each time.
Immunity is type specific, but some cross-protection may occur. Humoral antibodies to HSV-1 increase with age, starting at childhood during which HSV-1 is usually acquired. Antibodies to HSV-2 appear in sexually mature adults, correlating with their degree of sexual activity. The antibodies usually do not prevent recurrence of disease, but reduce the severity of clinical disease. Immunity is incomplete, and both reinfection and reactivation occur in the presence of circulating antibodies. CMI plays an important role in conferring immunity to HSV infection and facilitates recovery from the infection. Hence, the virus tends to cause more frequent and severe infections in the patients with altered CMI, such as HIV and other CMI-deficient diseases.
Clinical Syndromes
Herpes simplex virus causes a wide variety of clinical manifestations. The clinical manifestations depend on (a) the age of patient, (b) immune status of the host, (c) previous immunity of the patient to autologous or heterologous viruses, (d) antigenic type of the virus, and (e) anatomical site of involvement. Generally, HSV-1 produces the lesions above the waist, and HSV-2 produces lesions below the waist. HSV-1 infection is normally associated with orofacial infections and encephalitis, whereas HSV-2 is associated with genital infections. Primary infection with either virus is typically associated with systemic signs, prolonged duration, increased severity of illness, and more complications.
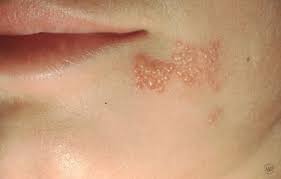
Herpes Simplex
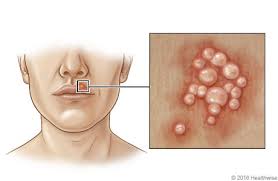
Herpes Simplex
HSV-2 infections
HSV-2 causes (a) genital herpes, (b) neonatal infection, and
(c) aseptic meningitis.
Genital herpes: Genital herpes is mostly caused by HSV-2 but
can also be caused by HSV-1. The latter causes less than 10% of
genital infections. Most primary genital infections are asymptomatic.
The clinical manifestations of primary genital herpes
caused by HSV-1 and HSV-2 are similar, but recurrences are
more common with HSV-2. In symptomatic men, the herpetic vesicles appear in the
glans penis, the prepuce, shaft of the penis, and sometimes on
the scrotum, thighs, and buttocks. In both men and women, the primary infection may be associated
with constitutional symptoms, such as fever, headache,
malaise, and myalgia. In both the sexes, the ulcerative lesions
persist from 4 to 15 days until crusting and re-epithelization
occur. The virus continues to shed in the ulcerative lesions for
more than 12 days.
Neonatal Infection: Neonatal infection is a most serious and usually fatal disease caused mostly by HSV-2. It usually occurs due to shedding of HSV-2 from the cervix during vaginal delivery. It can also occur from an ascending in-utero infection. Since CMI is poorly developed in neonates, the virus causes a disseminated disease with involvement of liver, lung, as well as the organs of the CNS. The condition has a high mortality of 80%.
Aseptic meningitis: Aseptic meningitis may occur as a complication of genital HSV-2 infection.
Epidemiology
Herpes simplex viruses are distributed worldwide. HSV-1 infection is more common than HSV-2 infection. By the age of 30 years, 80% individuals in high socioeconomic status and 80% in a low socioeconomic status are seropositive. Serum antibodies to HSV-2 begin to appear at puberty, correlating with degree of sexual activity.
Herpes simplex virus infections are exclusively human diseases. Humans are the only natural reservoirs. No vectors are involved in transmission of the disease. An infected person is a lifelong source and reservoir of the virus. Vesicle fluid, saliva, and vaginal secretions are the important sources of infection for both types of HSV. Children are at risk for acquiring HSV-1 infection, whereas sexually active people are at increased risk to HSV-2 infection.
Laboratory Diagnosis
Specimens include saliva, vesicle fluid, conjunctival fluid, corneal scraping, skin swab, skin scrapings, and cerebrospinal fluid (CSF).
Light microscopy of the stained infected cell may show ballooning of cells, ground glass nuclei and eosinophilic intranuclear inclusions, and multinucleated giant cells. Electron microscopy can be used for direct demonstration of virions in the negatively stained smears of the clinical specimens.
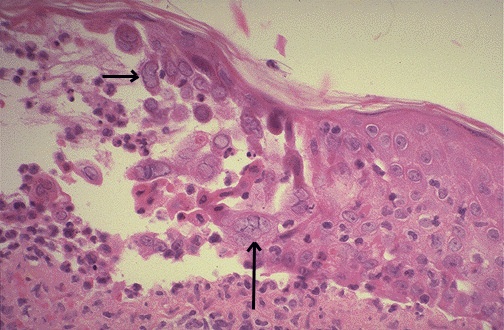
Microscopy of the lip showing typical pink homogenous intranuclear inclusions in the epithelial cells of the epidermis.
Direct enzyme immunoassay and direct fluorescent antibody test are useful to demonstrate HSV antigens directly in vesicular fluid, tissue smear, or biopsy.
Cell culture: scrapings of skin vesicles and mucosal lesions are collected, transferred immediately in a vial transport medium. After inoculation, HSV produces cytopathic effects (CPEs) within 1-3 days on HeLa cells, Hep-2 cells, and human embryonic fibroblasts. Some virus strains, particularly HSV-2, cause fusion of infected cells, leading to formation of syncytium.
Polymerase chain reaction (PCR) is a useful tool to distinguish HSV-1 from HSV-2. Isolated HSV can be typed by biochemical, immunological, and molecular methods.
Serodiagnosis is of little value for diagnosis of primary HSV infection. It is mainly used for epidemiological studies. It is used only to determine postexposure to HSV.
Treatment
Treatment with specific antiviral chemotherapy is used to (a) prevent disease and recurrence, (b) treat the infection, and (c) to reduce the clinical course of infection. Acyclovir: It is a synthetic acyclic purine nucleotide analog, which is most commonly used to treat HSV infection.
Prevention and Control
Prevention of genital HSV infection is difficult because most transmission occurs during subclinical viral shedding. Nevertheless, abstinence from sexual intercourse while the patients have prodromal symptoms or lesions or use of condoms may be useful.
Prevention of transmission of HSV from mother to infant is also difficult due to presence of asymptomatic primary or recurrent genital infection. In such infected mothers, transmission can be prevented by avoiding vaginal delivery and instead delivering by caesarian section. At present, no vaccine is available for use against HSV.
Herpesvirus Simiae: B Virus
Herpesvirus simiae is similar to HSV in many properties. These two viruses are antigenically related, though the antibody against HSV does not protect against herpesvirus simiae infection. Herpesvirus simiae in monkeys usually causes asymptomatic infection. In symptomatic cases, it is associated with formation of vesicles on the buccal mucosa. The lesion ulcerates, shedding the viruses in the ulcer exudate.
Varicella Zoster Virus
Varicella zoster virus (VZV) causes two distinct clinical entities in humans: (a) chickenpox (varicella) and (b) herpes zoster or shingles. Chickenpox is acquired by transmission from an infected host to a susceptible host, whereas herpes zoster occurs as a result of reactivation of the latent virus.
The virus has the smallest genome of all the human herpesviruses. It is an enveloped, dsDNA virus showing many similarities with the HSV.
Primary VZV infection occurs in humans when the virus comes into contact with the mucosa of the respiratory tract or conjunctiva. From these sites, the virus enters the blood stream and lymphatic system to the cells of the reticuloendothelial system. After 11-13 days, a secondary viremia occurs and the virus spreads throughout the body and to the skin. In tissues, VZV spreads from cell to cell via direct contact to produce its effects. After primary infection, the virus migrates along the sensory nerve fibers to the satellite cells of the dorsal root ganglia or cranial nerve ganglia, where it becomes latent. This latency may be permanent, or the virus may become reactivated in old adults or in patients with impaired cellular immunity. On reactivation, the virus replicates and spreads along the nerve fibers to the skin, known as herpes zoster or shingles.
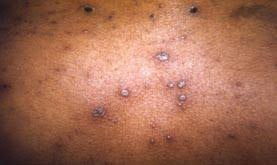
Chickenpox
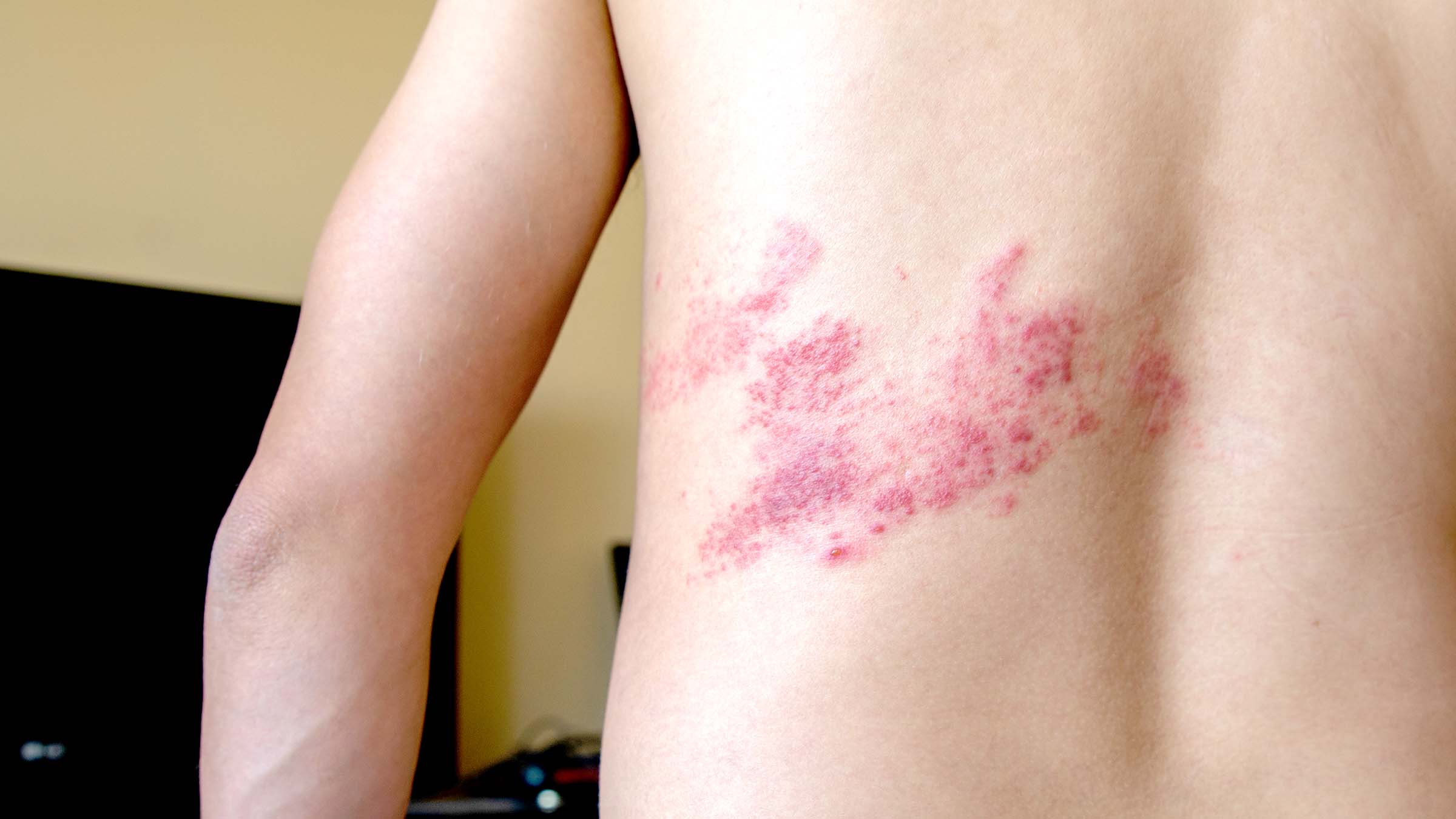
Shingles
CMI is important in controlling the infection. It limits the progression of the disease and results in early resolution of the disease. The virus causes a disseminated life-threatening and more serious disease in immunocompromised patients with a deficient CMI.
The Chickenpox syndrome
Varicella (chickenpox) Varicella is one of the five childhood exanthemata along with measles, rubella, rubeola, and fifth disease. Chickenpox is a benign illness of the childhood, which is characterized by an exanthematous varicella rash that occurs following infection with VZV. Incubation period is about 2 weeks. The condition is normally asymptomatic. In symptomatic cases, the condition manifests as fever and maculopapular rash that progresses within a few hours to thin-walled vesicle on an erythematous base. This vesicle, which is the hallmark of chickenpox, is characteristically surrounded by a red ring.
Primary infection in adults is generally more severe than in children. The vesicles heal more slowly; secondary bacterial infections and scarring are more common. The accompanying fever is more prolonged and higher. Interstitial pneumonia, Guillain–Barre syndrome, and Reye’s syndrome may occur in some of the patients.
Herpes Zoster (Shingles)
Herpes zoster is a recurrence of latent varicella infection acquired many years earlier. This occurs due to reactivation of the VZV, which has remained latent in one or more sensory ganglia following primary varicella many years earlier. The viruses travel down along the sensory nerve to produce painful vesicles in the areas of the skin (dermatome) innervated by the nerves from the affected ganglia. Severe pain in the area innervated by the nerve preceding the appearance of chickenpox-like lesion.
The accompanying pain and herpetic neuralgia is very much severe for up to a few weeks and occurs in about half of the patients over 60 years of age. The pain may persist for months, which may even require surgical ablation of the ganglion.
Chickenpox is exclusively a human disease. No animal reservoirs are present. A chickenpox or herpes zoster patient is the source of infection.
Laboratory Diagnosis
Specimens include skin lesion, respiratory secretions, or organ biopsy.
Diagnostic techniques include direct antigen detection, isolation of the virus and serodiagnosis.
Treatment
Antiviral drugs are available for treatment of VZV infections. These are acyclovir, famciclovir, and valacyclovir. Treatment with these agents is usually recommended for adults and immunocompromised patients with varicella infection and for patients with herpes zoster infections.
Epstein-Barr Virus
Epstein-Barr virus (EBV) or human herpesvirus 4 (HSV-4) is the causative agent of infectious mononucleosis. Epstein-Barr virus is also the first virus known to be associated with human malignancies, such as Burkitt's lymphoma, other B-cell lymphoma, and nasopharyngeal carcinoma.
Morphology
Epstein-Barr virus is an enveloped DNA virus. It consists of a genome, a capsid, and an envelope. The genome consists of a 172 kbp, linear dsDNA. It is surrounded by an icosahedral capsid composed of capsomeres. The viral capsid antigen (VCA) is the most important antigen present in the capsule and is of diagnostic importance.
Epstein-Barr viruses are sensitive to the action of ether and bile salts. They are relatively fragile and do not survive for a longer period outside the human body fluids.
Pathogenesis and Immunity
Epstein-Barr virus infection first occurs in the oropharynx and then spreads to the blood causing infection of B lymphocytes. The infection is most commonly transmitted through infected saliva often as a result of kissing. Therefore, the infectious mononucleosis is also commonly called as the kissing disease.
The virus infection induces a strong immune response, comprising circulating antibodies against many virus-specific proteins, cell-mediated immune responses, and production of lymphokines. The humoral immunity is characterized by the appearance of IgM antibodies. These antibodies against viral membrane antigen confer lifelong immunity against the second attack of infectious mononucleosis. The CMI plays an important role in controlling chronic infection and limiting primary infection.
Clinical signs and syndromes
EBV is associated with (a) infectious mononucleosis, (b) EBVinduced tumors, and (c) EBV infection in immunocompromised host.
Infectious mononucleosis: This is a clinical syndrome that represents the immunopathogenic response of the host to infection with EBV. It is the classic syndrome associated with primary EBV infection in adolescents and young adults. EBV is the causative agent in approximately 90% of cases of acute infectious mononucleosis. Cytomegalovirus (CMV) is most commonly associated with EBV-negative cases of infectious mononucleosis.
Epstein-Barr virus-induced tumors Epstein-Barr virus infection is associated with a number of tumors. Endemic Burkitt's lymphoma caused by EBV is a poorly differentiated monoclonal B cell lymphoma of the jaw. It is the most common tumor of childhood in Africa associated with both EBV and falciparum malaria.
Epstein-Barr virus infection in immunocompromised host The virus causes most severe diseases in patients who are immunocompromised. In these patients, the EBV causes several syndromes and proliferation disorders including Duncan syndrome, ataxia telangectasia and Wiskott-Aldrich syndrome.
Epidemiology
Epstein-Barr virus infection occurs worldwide, but since it is not a reportable infection, the exact prevalence of infection is not known.
Epstein-Barr virus infection is exclusively a human disease. Humans are the only known reservoirs of the virus. It is present in oropharyngeal secretion, saliva, peripheral blood, or lymphoid tissue of the infected human host. Saliva is the main source of infection.
Laboratory Diagnosis
Diagnosis of EBV-induced infectious mononucleosis
is based on the three classic criteria:
a. Presence of lymphocytosis.
b. Presence of at least 10% atypical lymphocytes in peripheral
blood smear.
c. Presence of heterophilic antibodies and antibodies to viral
antigens.
These criteria are supplemented by direct detection of viral
antigens or EBV genomes in clinical specimens.
Specimen include lymphoid tissues, nasopharyngeal carcinoma
tissue and saliva (for direct detection of viral antigens or EBV
genomes), and blood (for serological tests) and peripheral
blood (for blood smear).
Treatment
No specific treatment is available against acute infectious mononucleosis. Acyclovir has little activity against EBV; it may be useful in high doses for treatment of life-threatening EBV infections.
Prevention and Control
Avoidance of contact with saliva and avoidance of kissing children on the mouth are some of the preventive measures. No vaccine is available against EBV infection.
Cytomegalovirus
Cytomegalovirus or human herpesvirus 5 (HSV-5) is the causative agent of mononucleosis syndrome. It causes pneumonia and more serious diseases in immunocompromised patients. The name is derived from the word cytomegalo (large cell virus) and is derived from the swollen cells containing large multinuclear inclusions that characterize these infections.
Pathogenesis and Immunity
CMV is a lytic virus, which produces the CPE in vitro and in vivo. CMV infection is acquired by coming in contact with blood, tissue, and most body secretions containing viruses. The virus infects the epithelial cells of the salivary glands, causing a persistent infection and shedding of viruses in the salivary secretion. Activation and multiplication of the virus in the kidney and secretory glands facilitate secretion of CMV in urine and other body secretions including serum and milk.
In the infected cells, CMV infection produces characteristic enlarged cells with viral inclusion bodies. This histopathological change, most commonly referred to as owl's eye, is considered diagnostic of the CMV infections.
Immunity to CMV involves both humoral immunity and CMI. CMI is more important and essential (a) for resolution of infection and (b) also for controlling progression of CMV infection. The production of cytotoxic T cells against CMV is very crucial in CMI response to control the infection.
Epidemiology
CMV infection is worldwide. Humans are the natural hosts. An infected human is the only reservoir of CMV infection. No animal reservoirs are present for this virus. Cytomegaloviruses are found in the urine, blood, saliva, tears, throat swab, stool, semen, milk, amniotic fluid, cervical and vaginal secretions, and tissue obtained for transplantation. Saliva, tears, urine, and breast milk are the common sources of infection for baby or child. Cervical secretions are the source of transmission of infection to neonates. Blood, organ graft, and semen are the other sources of infection in adult population.
Laboratory Diagnosis
Different body secretions, such as saliva, urine, throat washing, blood, CSF, cervical secretions, and bronchoalveolar lavage fluid, and tissue bits are the specimens that can be used for the culture.
Treatment
Ganciclovir is the drug of choice for treatment of CMV. It is a nucleoside analog that inhibits DNA synthesis and also has activity against HSV, VZV, and human herpesviruses 7 and 8. Ganciclovir treatment is useful for gastrointestinal disease in patients who have received organ transplants and also those who are HIV positive.
Prevention and Control
sexual transmission can be limited by safe sexual practices, such as using condoms. Transmission of virus is also reduced by regular screening of blood and organ donors for CMV seronegativity. Prophylaxis with ganciclovir prevents reactivation of latent CMV infection in immunocompromised patients. No vaccine is available for CMV.
Adenoviruses
Adenoviruses cause a variety of sporadic and epidemic diseases in humans including acute respiratory disease, pharyngoconjunctival fever, epidemic keratoconjunctivitis, acute hemorrhagic cystitis, and more recently adenoviral infections in immunocompromised hosts.
Adenoviruses belong to the family Adenoviridae, which consist of a group of medium-sized, nonenveloped, double-stranded DNA viruses that multiply in the nucleus of the infected cell. The family consists of two distinct genera: Mastadenovirus and Aviadenovirus consisting of mammalian and avian adenoviruses, respectively.
Morphology
Adenoviruses show following characteristics:
- They are double-stranded DNA, nonenveloped viruses measuring 80-110 nm in diameter.
- Adenoviruses have a characteristic morphology. They are the only viruses having a fiber protruding from each of the 12 vertices of the capsid.
- An apical fiber, 9-31 nm in length and 62 kDa in molecular mass, projects from each penton. This apical fiber helps to bind specifically adenoviruses to receptor sites on the host cells. The fiber contains the viral attachment proteins and can act as a hemagglutinin.
Pathogenesis and Immunity
Adenoviruses are transmitted mainly by respiratory or feco- oral contact between humans. They infect the conjunctiva or the nasal mucosa. They may multiply in conjunctiva, pharynx, or small intestine, and then spread to preauricular, cervical, and mesenteric lymph nodes, where epithelial cells are infected. Adenoviruses may cause three different types of interaction with the infected cells. These are (a) lytic infection, (b) latent infection, and (c) transforming infection.
Lytic phase: Adenoviruses infect mucoepithelial cells in the respiratory tract, gastrointestinal tract, and conjunctiva or cornea, causing damage of these cells directly. After local replication of the virus, viremia follows with subsequent spread to visceral organs.
Latent infection: The adenovirus has a unique ability to become latent in lymphoid and other tissues, such as adenoids, tonsils, and Peyer's patches. Latent infections can be reactivated in patients infected with other agents or in the patients who are immunocompromised.
Oncogenic transformation: Some adenoviruses have the property for oncogenic transformation in rodent cells. However, oncogenesis of human cells has not been demonstrated.
Adenovirus infection is characterized by development of an effective and long-lasting immunity against reinfection. Both cell-mediated immunity (CMI) and humoral immunity are important. CMI plays an important role in limiting proliferation and outgrowth of adenoviruses.
Clinical Manifestations
Respiratory diseases
Acute respiratory disease: Fever, rhinorrhea, cough, and sore throat are the typical symptoms, which last for 3-5 days. This syndrome most often affects military recruits living in crowded conditions.
Pharyngoconjunctival fever: Fever, sore throat, coryza, and red eye are the classic presentations of the condition. These symptoms may precede ocular findings, or they may not be present. Acute conjunctivitis may occur as a separate entity with or without pharyngitis.
Epidemic keratoconjunctivitis
This is a highly contagious condition and has an insidious onset of unilateral red eye. Subsequently, both the eyes are involved. Patients complain of photophobia, tearing, and pain. Inflammation of the conjunctiva may persist for a week, accompanied with residual scarring and visual impairment.
Gastroenteritis and diarrhea
The enteric adenovirus infection is a common cause of infantile diarrhea in day-care centers. The condition manifests as fever and watery diarrhea, which resolves within 1-2 weeks.
Epidemiology
Adenovirus infections are found worldwide. Adenovirus infections are exclusively human infections. No animal reservoirs are present. Infected symptomatic as well as asymptomatic humans who excrete adenoviruses intermittently in their respiratory secretions and also in their stool are the sources of infection. Overcrowding, poor hygiene, and close contact facilitate transmission of infection. Adenovirus typically affects children, starting from infants to school-going ones, though children of any age may be affected.
Laboratory Diagnosis
Specimens used depend on the nature of the clinical illness caused by adenoviruses. These include throat swab, nasopharyngeal aspirate, bronchial lavage, conjunctival swab, corneal scraping, urine, and feces.
- Microscopy
- Direct antigen detection
- Isolation of the virus
- Serodiagnosis
Treatment
At present, no specific antiviral agents are available for treatment of adenovirus infections. Ribavirin has been used with variable success in treatment of adenovirus infections in immunosuppressed hosts.
Parvoviruses
Parvoviruses are the smallest of the DNA viruses belonging to the family Parvoviridae. They are icosahedral, nonenveloped viruses containing a single-stranded DNA.
The family Parvoviridae consists of three genera: Dependovirus, Parvovirus, and Erythrovirus.
Parvovirus B19
Parvovirus B19, or B19 virus, is the causative agent of erythema infectiosum, a mild viral illness of children
Morphology
B19 viruses are extremely small viruses, measuring 18-26 nm in diameter. They possess a nonenveloped, icosahedral capsid. The viral genome contains a single-stranded DNA measuring 4000-6000 bases in length. The genome is negative-strand DNA, but there is no virion. The genome encodes for many proteins, which include three structural, one major nonstructural, and several smaller proteins.
B19 virus is highly resistant to inactivation but can be inactivated by formalin, beta propiolactone, and oxidizing agents. The viruses withstand heating at 56oC for 30 minutes and are stable between pH 3 and 9.
Pathogenesis and Immunity
It has been demonstrated that the B19 virus first enters through the nasopharynx or upper respiratory tract and then spreads to the blood, causing viremia. The virus infects rapidly dividing erythrocyte precursors, such as bone marrow cells, erythroid cells from fetal liver, and erythroid leukemia cells, and destroys these cells after infection, thereby causing aplastic anemia. Infection of the endothelial cells in the blood vessels leads to erythema infectiosum.
Inside the red cells, the virus enters the nucleus, starts replicating, followed by killing of the red cells. The production of RBCs is stopped for approximately 1 week due to killing of the erythroid precursor cells by the viruses.
The initial stage is associated with flu-like illness caused by large viremia. The viruses are shed in the oral and respiratory secretions and even cross the placenta. Subsequently, viremia is controlled by the production of specific antibodies against B19 virus.
The disease exhibits two stages: initial stage is flu-like illness and second stage is appearance of rash and arthralgia. Host immunity to B19 virus infection is primarily antibody mediated.
Clinical Syndromes
B19 virus causes following clinical syndromes: (a) flu-like illness, (b) erythema infectiosum or fifth disease, (c) infection in pregnant women, and (d) chronic B19 infection.
- Flu-like illness characterised by Malaise, headache, myalgia, and rhinorrhea.
- Erythema infectiosum: The infection begins with nonspecific symptoms followed by appearance of a distinctive rash on fifth day of infection. A bright red rash develops on both cheeks that appear as they have been slapped. The rash then appears on the trunk, which spreads gradually toward the arms and legs. The condition usually subsides within 1-2 weeks.
- Infection in pregnant women: In nonimmune seronegative pregnant mothers, B19 virus infection is increasingly associated with risk for fetal death. The infection may cause severe anemia in the fetus and subsequently, the fetus may develop signs of high-output cardiac failure.
- Chronic B19 infection: This infection occurs in immunocompromised patients, such as patients with HIV, those receiving immunosuppressive therapy, and transplant patients. Chronic anemia, leukopenia, and thrombocytopenia are the common manifestations.
Epidemiology
B19 virus is distributed worldwide. Approximately, 90% of adults older than 60 years are seropositive in the United States. B19 virus infection is strictly a human disease. Humans are the only reservoir. Viruses are excreted in respiratory samples, which are the primary source of infection. The infected patient is contagious from 24 to 48 hours before developing prodromal syndrome
Laboratory Diagnosis
Demonstration of specific IgG and IgM antibodies in the serum is useful for diagnosis of erythema infectiosum caused by B19 virus. ELISA (enzyme-linked immunosorbent assay), RIA (radioimmunoassay), and IFA (indirect fluorescent antibody) for demonstration of IgG and IgM antibodies are available. In pregnant women, the serum positive for IgG and IgM antibodies indicates B19 virus infection within 7 days to 4 months and a possible risk to fetus.
No specific antiviral therapy is available for treatment of B19 virus infection.
A clinical-grade recombinant virus-like particle (VLP) vaccine has been developed to prevent human parvovirus B19 (HPVB19) infection
Picornaviruses
The family Picornaviridae is one of the largest families of viruses and includes a large number of very small ( pico: measuring small; rna: RNA virus) RNA viruses. They are nonenveloped viruses measuring 27-30 nm in size. The capsid is a naked icosahedral made up of 60 protein subunits. The genome consists of a single linear molecule of single-stranded RNA. The genome RNA is unusual, because it has a protein at the 5' end that serves as a primer for transcription by RNA polymerase.
Enteroviruses
Human enteroviruses consist of at least 72 serotypes, which include poliovirus types 1-3, coxsackieviruses A types 1-24, coxsackieviruses B types 1-6, echoviruses types 1-34, and enteroviruses 68-71.
Poliovirus
Poliomyelitis is an enteric infection caused by polioviruses transmitted by the fecal–oral route. The worldwide prevalence of poliomyelitis has decreased by more than 99% due to improved socioeconomic conditions and availability of vaccines.
Morphology
Poliovirus was the first animal virus to be purified and obtained in crystalline form. The viruses are spherical particles about 27 nm in diameter. The virion is composed of 60 subunits, each consisting of four viral proteins. The viral genome is a single-stranded positive-sense RNA, which can be directly translated by host ribosomes to form a polyprotein, which is divided into 11 different proteins.
Pathogenesis and Immunity
Poliovirus is transmitted by the fecal oral route on ingestion of contaminated water. The viral particles initially multiply in the nasopharynx and the gastrointestinal mucosa. The virions are resistant to acidity of stomach and to lytic activities of the protease and other enzymes of the intestinal tract and bile.
On entering the body, the virus infects and multiplies in the tonsils and Peyer's patch of the ileum. It then spreads to regional lymph nodes and enters the blood, causing a primary viremia. On continued infection and multiplication of virus in the reticuloendothelial cells, it invades the blood stream again and causes secondary viremia. During this period of viremia, the poliovirus crosses the blood-brain barrier or gains access to the brain by infecting skeletal muscle and traveling up on the nerves to the brain as in rabies virus.
It recognizes a receptor present on the anterior horn cells of (a) the spinal cord, (b) dorsal root ganglia, and (c) motor neurons. On combination at these sites, the poliovirus causes destruction of the motor neurons, anterior horn, and brain stem. The destruction of motor neurons leads to paralysis of the muscles innervated by those neurons.
The poliovirus also infects the brain stem, causing bulbar poliomyelitis associated with respiratory paralysis. Poliovirus causing the pathological changes in the central nervous system (CNS) is usually responsible for causing symptoms of poliomyelitis. Immune mechanisms do not play any role in pathogenesis of the disease.
Host immunity in poliomyelitis is mostly dependent on the humoral antibodies. Both the serum and secretory antibodies play an important role in conferring protection against poliomyelitis. Cell-mediated immunity plays little or very insignificant role in the immunity against poliovirus. Host immunity is type specific.
Postpoliomyelitis syndrome: This condition is sequelae of poliomyelitis, which may develop 20-40 years after infection with poliovirus. This condition is seen in 20-80% of patients who have recovered from poliomyelitis. Recurrence of weakness or fatigue is observed in this condition, and it usually involves the muscles that were initially affected by the poliovirus.
Epidemiology
Poliovirus mainly affects children. However, individuals of any age may also develop the disease. Following the widespread use of poliovirus vaccine in the mid-1950s, new cases of poliomyelitis declined dramatically in many industrialized countries. A global effort to eradicate polio began in 1988, led by the World Health Organization, UNICEF, and The Rotary Foundation. In 2015, cases decreased to 98 and further decreased in 2016 to 37 wild cases and 5 circulating vaccine-derived cases, but increased in 2019 to 175 wild cases and 365 circulating vaccine-derived cases.
In 2013, the Center for Disease Control received reports of 183 cases of polio in Somalia, 14 in Kenya and 8 cases in the Somali Region of Ethiopia, but Africa had no confirmed cases of wild poliovirus (WPV) since 2016. Cases of circulating vaccine-derived poliovirus type 2 continue to appear in several countries.
Natural infection occurs only in humans. Infected humans excreting poliovirus in their stool are the major reservoir of infection. Infected stool containing poliovirus is the major source.
Laboratory Diagnosis
Specimens - include stool, throat swab, and cerebrospinal fluid (CSF) are the specimens used for isolation of the viruses. Viruses can be isolated from feces for more than 30 days during the illness and from the throat swab during the first few days of the illness. The virus is isolated rarely from the CSF specimens.
Microscopy of the CSF shows a predominantly lymphocytic pleocytosis with the presence of 25-500 cells/mm3. The virus is rarely demonstrated in the CSF.
Isolation of the virus: Virus isolation from feces and throat swab is carried out by cultivation on monkey kidney, human amnion, HeLa, Hep-2, Buffalo green monkey (BGM), MRC-5, and other cell cultures.
Serodiagnosis is based on demonstration of a fourfold increase in the antibody titer of the serum collected at the time of acute illness and the period of convalescence.
No antivirals are available for the treatment of poliomyelitis.
Poliovirus vaccines are the key component in prevention of polio and have played an important role in the effort to eradicate polio worldwide.
Coxsackieviruses
Coxsackieviruses are so named, because the viruses were first isolated in Coxsackie village in New York, United States, by Dandruff and Sickel in the year 1985. These viruses based on the pathological changes produced in suckling mice are classified into two groups: coxsackieviruses A and coxsackieviruses B.
The structure and morphology of the coxsackieviruses and the RNA genome are similar to those of poliovirus. But unlike poliovirus, they can infect mammals other than primates. Replication cycle is similar to that of poliovirus.
Coxsackie A viruses show tropism for skin and mucous membranes, whereas coxsackie B viruses show predilection for visceral organs, such as liver, heart, pancreas, and pleura. Both the viruses infect anterior horn cells of the motor neurons and meninges and cause paralysis. They replicate first in the oropharynx and gastrointestinal tract from where they spread by the blood circulation. Host immunity is IgG-antibody mediated and is type specific.
Diseases specifically caused by coxsackie A viruses include:
Herpangina: This condition is caused by coxsackie A virus serotypes. The infection is most commonly seen in children between 1 and 7 years. The symptoms include sudden onset of fever, sore throat, and difficulty in swallowing.
Hand-foot-and-mouth disease: This is a vesicular exanthema usually caused by coxsackievirus serotypes 5 and 16. This is mainly a disease of children, seen most commonly in patients younger than 10 years.
Pleurodynia: This condition, also known as epidemic myalgia or Bornholm disease, is caused by coxsackie B virus serotypes 3 and 5. This condition is an acute illness, which manifests with a sudden onset of fever accompanied by muscular pain and pain in the chest and abdomen.
Myocarditis: This is a serious condition caused by coxsackie B virus, mostly in newborn infants. Shortness of breath, dull or sharp chest pain, and fever are the common manifestations of the condition.
Echoviruses
Echoviruses were originally isolated from the feces of an individual who had no clinical illness and caused a cytopathic effect in the cell culture. The prefix ECHO is an acronym for enteric cytopathogenic human orphan viruses (ECHO viruses).
The echoviruses resemble other enteroviruses in their properties.
Most of the echoviruses cause asymptomatic infections in humans, but some of them have been associated with many clinical syndromes. Nonspecific febrile illness associated with rash, headache, and common-cold-like symptoms is caused by many serotypes of echoviruses. Aseptic meningitis is also caused by echoviruses. Some strains of echovirus have been associated with gastroenteritis and respiratory diseases in children.
Laboratory diagnosis of echovirus infection is made by isolation of viruses from feces, throat swab, or CSF. They are cultured using human diploid embryonic lung fibroblast and human rhabdomyosarcoma cell lines. The growth of the virus is detected by cytopathic changes, which is similar to that of other coxsackieviruses.
Rhinoviruses
Rhinoviruses are the most important causative agents of common cold and upper respiratory tract infections. Earlier, many viruses isolated from the cases of common cold were known as common cold viruses, Salisbury viruses, or Muri viruses. Now all these viruses have been given the name rhinoviruses (rhino referring to the organ specifically affected).
Rhinoviruses are small RNA viruses, morphologically resembling other picornaviruses. They grow better in vivo at a temperature of 30oC than at 37oC, a property that contributes partially for predilection of these viruses for the cooler environment of the nose and conjunctiva. The rhinoviruses have been classified into more than 102 serotypes on the basis of a type-specific antigen in their capsids.
Infection is transmitted to other susceptible human hosts by nasal secretions expelled from the nose of a patient during sneezing and coughing. Infection can be initiated by as little as one infectious viral particle.
The infection occurs mostly by multiplication of the virus in the nose. The onset and severity of the symptoms usually correlate with the quantity of viruses excreted in the nasal secretion and the time of viral shedding. Infected cells release bradykinin and histamine, which cause a running nose. The progression of infection is limited by an interferon which is produced in nasal secretion in response to the nasal infections.
Host immunity in rhinoviruses is characterized by both humoral and cell-mediated immunities. Humoral immunity is characterized by the production of both nasal secretory IgA and serum IgG antibodies within a week of infection. The cell- mediated immunity does not play any important role in controlling rhinovirus infection.
Orthomyxoviruses
The term myxovirus was coined for a group of enveloped RNA viruses that have the ability to adsorb onto mucoprotein receptors on erythrocytes, causing hemagglutination. It included influenza, mumps, parainfluenza, and Newcastle disease viruses.
Influenza Viruses
Influenza viruses are classic respiratory viruses. They cause influenza, an acute respiratory disease, with well-defined systemic symptoms. Influenza is an acute infectious disease of the respiratory tract that occurs in sporadic, epidemic, and pandemic forms.
Morphology
Influenza viruses are spherical or filamentous, enveloped particles 80-120 nm in diameter. They are composed of a characteristic segmented single-stranded RNA genome, a nucleocapsid, and an envelope. The viral genome is a single-stranded antisense RNA. The viral RNA has a molecular weight of 5 million daltons and a length of 13,600 nucleotides. Characteristically, it is segmented and consists of seven or eight segments. These segments code for different proteins which are NS1, NS2, NP, M1, M2, M3, HA, and NA. The genome is present in a helically symmetric nucleocapsid surrounded by a lipid envelope. The envelope has an inner membrane protein layer and an outer lipid layer.
Viral Replication
Influenza virus, hepatitis delta virus, and retroviruses are the only RNA viruses that have an important stage of their replication cycle in the nucleus. Infection of the host cell begins by adsorption of the cell by influenza virus, which is mediated through the HA. HA is first cleaved by an extracellular protease to a modified HA that actually mediates the attachment of the virus to the cell surface. In the nucleus of the host cell, the virion RNA polymerase transcribes the eight-genome segments into eight viral mRNAs. Most of the viral mRNAs, however, move out of the nucleus into the cytoplasm, where they are translated into viral proteins. Some of the viral mRNAs continue to remain in the nucleus and serve as the templates for synthesis of the negative-strand RNA genomes for the progeny virions.
Antigenic and genomic properties
The surface antigen, or 'viral' antigen,
or 'V antigen' is composed of two virus-encoded proteins,
HA and NA, which are the type-specific antigens.
Hemagglutinin: HA is a trimer and is composed of two polypeptides,
HA1 and HA2, responsible for hemadsorption and hemagglutination. The hemagglutinin consists of 500 spikes. The distal end,
which contains five antigenic sites (designated as HA1-HA5), is
responsible for binding of virion to host cells.
Neuraminidase: The NA is a glycoprotein and tetramer. It consists of 100 mushroom-shaped spikes. The mushroom-shaped NA is inserted into the virus membrane by its hydrophobic tail end. The distal end contains antigenic as well as enzymatically active sites. The NA causes hydrolysis of N-acetyl neuraminic acid or sialic acid residues present on the glycoprotein receptors on red cells, hence causes elution or detachment of the cells adsorbed to virion particles. The neuraminidase also degrades the mucus layer, thereby exposing the epithelial membrane of the respiratory tract for infection by the virus.
Antigenic variations
Antigenic variation is a unique feature of influenza virus. The surface antigens HA and NA show variations and are primarily responsible for antigenic variations exhibited by influenza viruses. The internal RNP antigen and M protein are stable, hence do not contribute to the antigenic variations. Antigenic variations are of two types: antigenic shift and antigenic drift.
Gene reassortment
Because the influenza virus genome is segmented, genetic reassortment can occur when a host cell is infected simultaneously with viruses of two different parent strains. This process of genetic reassortment accounts for the periodic appearance of the novel types of influenza A strains that cause influenza pandemics.
Influenza viruses of animals, such as aquatic birds, chickens, swine, and horses show high host specificity. These animal viruses are the source of the RNA segments that encode the antigenic shift variants that cause epidemics among humans. For example, if a person is infected simultaneously by an avian and human influenza strains, then it is possible that a genetic reassortment could occur in infected cells in humans. The reassortment could lead to emergence of a new human influenza A virus, the progeny of which will contain a mixture of genome segments from the two strains (e.g., a new variant of human influenza A virus bearing the avian virus HA)
Many studies have conclusively demonstrated that the aquatic birds (such as water fowl) are a common source of these new genes. The pigs act as mixing vessels, where these virulent genes of water fowl mix with the genome of influenza virus giving rise to new variant of influenza virus.
Pathogenesis and Immunity
Inhaled influenza viruses reach lower respiratory tract, tracheobronchial tree, the primary site of the disease. They attach to sialic acid receptors on epithelial cells by HA present on the viral envelope.
Neuraminidase of the viral envelope may act on the N-acetyl neuraminic acid residues in mucus to produce liquefaction. In concert with mucociliary transport, this liquefied mucus may help spread the virus through the respiratory tract. Infection of mucosal cells results in cellular destruction and desquamation of the superficial mucosa. The resulting edema and mononuclear cell infiltration of the involved areas are accompanied by symptoms including nonproductive cough, sore throat, and nasal discharge. Although the cough may be striking, the most prominent symptoms of influenza are systemic: fever, muscle aches, and general prostration. The virus remains localized to the respiratory tract; hence viremia does not occur.
Occasionally, in patients with underlying heart or lung disease, the infection may extensively involve the alveoli, resulting in interstitial pneumonia, sometimes with marked accumulation of edema and lung hemorrhage. Pure viral pneumonia of this type is a severe illness with a high mortality.
In most cases, however, pneumonia associated with influenza is caused by bacteria, principally pneumococci, staphylococci, and Gramnegative bacteria. These bacteria can invade and cause disease, because the preceding viral infection damages the normal defenses of the lung.
Antibody is the primary defense in immunity to reinfection. IgA antibody, which predominates in upper respiratory secretions, is less persistent than secretory IgG, but contributes to confer immunity. Secretory IgG antibody, which predominates in lower respiratory secretions, appears to be the most important. Antibodies provide long-lasting immunity against the infecting influenza strain. Only antibodies directed against HA is able to prevent infection.
Immunity to influenza virus is strain-specific and lasts for many years. Recurrent cases of influenza are caused primarily by antigenically different strains. The role of cell-mediated immunity in conferring protection against influenza is not clear.
Clinical Syndrome
Incubation period is short (1-3 days). The classic influenza syndrome is a febrile illness of sudden onset, characterized by tracheitis and marked myalgias. Headache, chills, fever, malaise, myalgias, anorexia, and sore throat appear suddenly. The body temperature rapidly rises to 101–104°F (38.3–40.0°C) and respiratory symptoms ensue. Nonproductive cough is characteristic. Sneezing, rhinorrhea, and nasal obstruction are common. Patients may also report photophobia, hoarseness, nausea, vomiting, diarrhea, and abdominal pain.
Some patients may exhibit symptoms including predominantly sneezing, nasal obstruction, and nasal discharge (common cold); nasal obstruction, discharge, and sore throat (upper respiratory illness); sore throat with erythema (pharyngitis); hoarseness (laryngitis); or cough (tracheobronchitis). Fever may be absent.
Secondary bacterial infections: Life-threatening influenza is often caused by secondary bacterial infections with staphylococci, pneumococci, and Haemophilus influenzae. Pneumonia may develop as a complication and may be fatal, particularly (a) in elderly persons above 60 years with underlying chronic disease, (b) in people with impaired resistance (chronic cardiorespiratory disease, renal disease, etc.), and (c) in pregnant women.
Central nervous system complications: Guillain–Barre syndrome characterized by encephalomyelitis and polyneuritis is a rare complication of influenza virus infection. This condition was documented in the United States in the year 1976, following extensive vaccination with inactivated H3N2 influenza virus.
Other complications: Reye's syndrome is a noted complication of influenza B infection. The condition is seen most commonly in young children and is associated with degenerative changes in the brain, liver, and kidney.
Epidemiology
Influenza viruses cause epidemic, endemic, and pandemic influenza. Influenza A virus causes epidemics and occasionally pandemics; influenza B virus only causes epidemics; and influenza C viruses only cause minor respiratory illness and do not cause any epidemics.
Infected humans are the main reservoir of infections for influenza A virus. Respiratory secretions of infected persons are the important source of infection. The virus is excreted in respiratory secretions immediately before the onset of illness and for 3-4 days thereafter.
Wild aquatic birds are known reservoirs of influenza A. They secrete the viruses in their feces, which contaminates ponds and lakes. The virus is spread from person-to-person primarily by air-borne respiratory droplets released during the acts of sneezing and coughing.
Treatment
Amantadine and rimantadine are the specific antiviral agents available for treatment of influenza. These drugs are effective against influenza A virus but not against influenza B virus.
Prevention and Control
Prevention involves Immunoprophylaxis by vaccines and Chemoprophylaxis.
H5N1 bird flu
The H5N1 flu, caused by an avian subtype influenza virus, which has been associated with bird flu in the domesticated birds, can be transmitted from birds to humans. The H5N1 was first described in Hong Kong in 1997. Human infections caused by this virus were established in only 18 individuals of which six died. Since then, sporadic cases of H5N1 infection continued to be described in southern China.
There is no conclusive evidence to show the human-to-human transmission of H5N1. However, scientists are concerned that a slight mutation could convert H5N1 to a strain that would be easily transferred from human-to-human.
An epidemic of bird flu occurred in domesticated birds in Southeast Asia, primarily Vietnam, in January 2004. More than 240 human cases have been documented and more than 140 persons have died due to the poultry outbreaks and bird-to-human transmission.
Another avian subtype, H9N2, was described in two young children in March 1999. However, after that despite concern, no further outbreak of H9N2 infection has been documented.
H1N1 Swine Flu
emerged in 2009 to produce the first human influenza pandemic of the twenty-first century. Within 1 year, this virus spread to 214 countries and caused more than 18,000 confirmed deaths worldwide. small percentage of those affected were found to develop pneumonia or acute respiratory distress syndrome. The number of cases decreased greatly by May 2010, and the pandemic was officially declared to be at an end in August 2010.
Diagnosis of H1N1 influenza involves testing of samples like nasopharyngeal swabs or oropharyngeal swabs. Real time RT-PCR for viral nucleic acid is the recommended method for its diagnosis, and other rapid tests like antigen detection were not found to be useful.
The treatment for severe cases of H1N1 influenza is the neuraminidase inhibitor, oseltamivir.
Two types of influenza vaccines have been developed against H1N1 influenza trivalent inactivated vaccine and a live attenuated influenza vaccine, which can be administered intranasally.
Paramyxoviruses
Paramyxoviruses are roughly spherical-shaped viruses and usually vary in size from 100 to 300 nm. Sometimes, long filaments and giant forms of the virus measuring up to 800 nm are also found. These viruses consist of a negative-sense single-stranded RNA genome enclosed in a helical nucleocapsid surrounded by a pleomorphic envelope.
Paramyxoviruses resemble orthomyxoviruses in morphology but are larger, surface spikes are different, and their genomes are not segmented
Measles Virus
Measles is one of the five classic exanthematous diseases of the childhood; others being chickenpox, rubella, roseola, and fifth disease. Measles is a highly communicable acute viral disease characterized by fever, conjunctivitis, and pathognomonic Koplik's spots.
Morphology
Measles virus is spherical, but is often pleomorphic, measuring 120-250 nm in diameter. It contains a negative-sense RNA genome. The helical nucleocapsid is surrounded by an envelope carrying H and F protein on its surface. The virus causes hemagglutination of monkey erythrocytes, but it is not followed by elution as the virus does not produce any neuraminidase activity.
The measles virus has only one serotype and infects only humans, not any other mammals. The virus is antigenically uniform; it shares antigens with canine distemper virus.
Pathogenesis and Immunity
Measles is highly contagious and is spread from person to person by aerosols. It enters the susceptible host by the respiratory route.
The virus initiates infection and replicates locally in the trachea and bronchial epithelial cells of the respiratory tract. After 2-4 days, the virus spreads systemically in lymphocytes. dissemination of the virus causes infection of the conjunctiva, respiratory tract, urinary tract, lymphatic system, blood vessels, and the central nervous system (CNS). The characteristic rash seen in measles is caused primarily by cytotoxic T cells attacking the measles virus-infected epithelial cells in the skin.
Measles causes immunosuppression, characterized by decrease in eosinophils and lymphocytes (both B and T cells) and depression of their response to activation by mitogens. Cell-mediated immunity (CMI) plays an important role to control measles infection. Therefore, measles virus in individuals with deficiency in cellular immunity causes progressive and often fatal giant cell pneumonia without a rash. The antibodies do not have any role in conferring protection against measles virus, because the viruses spread from cell to cell. However, maternal antibodies in infants protect against measles during first 6 months of life.
Clinical Syndromes
the virus causes clinical syndromes including (a) measles, (b) atypical measles, and (c) subacute sclerosing panencephalitis.
Measles: Incubation period varies from 8 to 12 days. Measles is a highly contagious febrile illness. The prodromal phase is characterized by high fever, malaise, anorexia, conjunctivitis, cough, and coryza. Koplik's spot is the typical pathogenic lesion found in the mucous membrane. These are bluish gray specks or grain substance on a red base, which usually appear on the buccal mucosa opposite the second molar.
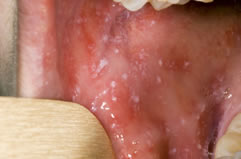
Koplik's spots
An erythematous maculopapular rash appears within 12-24 hours of appearance of the Koplik's spots. The rash usually begins on the face, then spreads extensively and appears on the trunk, extremities, palms, and soles and lasts for about 5 days. Desquamation of the rashes except those of palms and soles may occur after 1 week. Patients appear highly sick during the first or second day of the appearance of the rash.
Complications of measles:
Complications include otitis
media, bronchopneumonia, laryngotracheobronchitis (croup),
and diarrhea. Bronchopneumonia is the most serious complication
and is responsible for 60% of deaths caused by measles virus.
Hepatitis, encephalitis, and SSPE are the rare complications.
Atypical Measles:
Atypical measles is a syndrome that has been described in people who were infected with measles virus after immunization with the older, killed measles vaccine used during 1963-1977. The condition is characterized by a prolonged high fever, pneumonitis, and the rash.
Subacute sclerosing panencephalitis:
The subacute sclerosing panencephalitis (SSPE) is a degenerating disease of the CNS caused by persistent measles infection. It is the most serious and late neurological sequelae of measles that affects the CNS. The disease is characterized by the development of behavioral and intellectual deterioration and seizures after many years (mean incubation period is 10.8 years) of infection by measles. The condition occurs in about seven in every 1 million patients. The condition occurs most commonly in children who were initially affected when they were younger than 2 years. The condition is associated with the presence of an extremely high measles antibody titer in the blood and cerebrospinal fluid (CSF).
Epidemiology
Measles is a disease reported throughout the world. Epidemics of measles occur every 2-3 years. Approximately, 30 million cases of measles are reported annually, most cases being from Africa. The condition has also been well documented in the America, Europe, Eastern Mediterranean region, Western Pacific region, and Southeast Asia.
Children with immunodeficiency due to leukemia, corticosteroid therapy, or HIV are at increased risk for infection regardless of their status of immunization. Unvaccinated people are also at risk.
Laboratory Diagnosis
The clinical manifestations of typical measles cases are so characteristic that the diagnosis is self-evident. The laboratory diagnosis is frequently helpful to diagnose atypical measles and to differentiate from rubella.
Respiratory specimens, conjunctival specimens, urine, blood, and brain tissues are the frequently used specimens. The respiratory specimens and blood collected during the prodromal stage and the period following until 2 days after the appearance of the rash are the specimens of choice for isolation of viruses by culture.
Demonstration of multinucleated giant cells, measuring up to 100 nm in diameter, in Giemsa-stained smears is diagnostic of measles.
Measles antigen can be detected in nasal secretions, pharyngeal secretions, or in urinary sediments by direct immunofluorescence antibody test.
The measles virus is difficult to isolate, but it can be grown in primary human or monkey kidney cell cultures. The virus produces cytopathic effects (CPEs) very slowly, usually 1 week after innoculation.
On serodiagnosis, demonstration of more than fourfold rise in IgG antibody titer between acute and convalescent sera confirms diagnosis of measles. IgG antibodies usually appear 4 days after the onset of rash and persist for a longer period even after the patient has been cured of the illness.
Treatment
Ribavirin given either intravenous or in aerosol form is being now evaluated to treat severely affected adults and immunocompromised individuals with acute measles or SSPE.
Prevention and Control
Measles vaccine along with mumps and rubella (MMR) vaccine is currently used for universal immunization of children. Serum human gamma globulin, if administered within 6 days of exposure to measles, may prevent or attenuate the disease.
Parainfluenza Virus
Human parainfluenza viruses (HPIVs) are the pathogens that primarily affect young children; in whom the viruses cause upper and lower respiratory tract infections.
HPIVs are pleomorphic viruses measuring 150-200 nm in diameter. The virus contains a single-stranded, nonsegmented, negative- sense RNA genome with nucleoproteins P and L. It is surrounded by a helical nucleocapsid, which contains glycoprotein spikes. The surface spikes consist of H, N (neuraminidase), and F proteins. Both H and N proteins are present on the same spike, whereas the F protein is present on a separate spike. The F protein mediates the formation of multinucleated giant cell.
Pathogenesis and Immunity
The virus adsorbs to the respiratory epithelial cells by specifically combining with neuraminic acid receptors in the cell through its hemagglutinin. Subsequently, the virus enters the cells following fusion with the cell membrane. The virus replicates more rapidly than mumps and measles viruses in the cell cytoplasm and causes formation of multinucleated giant cells. These giant cells, each of which contains two to seven nuclei, usually develop late in the infection. The virus also causes the formation of single and multilocular cytoplasmic vacuoles and basophilic or eosinophilic inclusions.
Humoral immunity plays a major role in defense against HPIVs. The antibodies are produced against both surface glycoproteins (HN and F) of the virus.
Clinical Syndromes
Human parainfluenza viruses cause croup, pneumonia, bronchiolitis and tracheobronchitis, and some other infections.
Croup: also called laryngotracheobronchitis. It is a heterogeneous group of illnesses that affects the larynx, trachea, and bronchi. The condition manifests as fever, cough, laryngeal obstruction, and expiratory stridor.
Pneumonia: HPIV-1 and HPIV-3 are responsible for most cases of human parainfluenza pneumonia. Fever, rales, and evidence of pulmonary consolidation are the common symptoms.
Other infections: Otitis media, pharyngitis, conjunctivitis, and coryza are the other infections caused by HPIV.
Epidemiology
Human parainfluenza viruses are ubiquitous. Parainfluenza viruses cause disease exclusively in humans. No animal reservoirs are present. Respiratory secretions from the infected humans are the source of infection.
Laboratory Diagnosis
Respiratory specimens include nasopharyngeal aspirations, nasal washings, and nasal aspirations.
The ELISA, immunofluorescence assay, and fluoroimmunoassays are used to detect HPIV antigen directly in urine specimens. These methods are sensitive and specific.
The virus can be isolated from clinical specimens by culture in PMK and LLC-MK2 cell lines. The CPEs are rarely demonstrated during primary isolation of the virus in tissue culture.
Serodiagnosis: Hemagglutination inhibition, neutralization, ELISA, and Western blot are frequently used antibody-based serological tests for diagnosis of HPIV infection.
Ribavirin has been shown to be effective against HPIV infection in vitro. Uses of ribavirin aerosols or systemic therapy for treatment of HPIV infection in children and adults who are severely immunocompromised have shown mixed results with uncertain clinical benefit.
Live attenuated vaccine is available. Field trials of formalinkilled whole HPIV (HPIV-1, HPIV-2, and HPIV-3) have proved to be ineffective in children. These vaccines failed to protect against natural infection by parainfluenza virus.
Mumps Virus
Mumps is an acute infectious disease of children, characterized by acute, nonsuppurative, painful swelling of the salivary glands, caused by mumps virus.
Mumps virus is a typical paramyxovirus containing a singlestranded, negative-sense RNA surrounded by an envelope. It has two major surface glycoproteins: (a) one with both hemagglutinin and neuraminidase and (b) the other with cell-fusion protein. The hemagglutinin agglutinates the RBCs of fowl, guinea pigs, humans, and many other species. The hemagglutination is followed by hemolysis and elution at 37oC.
Pathogenesis and Immunity
Infection by mumps virus begins after the entry of the virus into the respiratory tract. The virus then replicates locally and disseminates by blood circulation to target tissues, such as the CNS and salivary glands, particularly the parotid glands. The virus replicates in these target tissues and then causes a secondary phase of viremia. The virus is spread by viremia throughout the body to kidneys, testes, ovary, pancreas, and other organs. Infection of the CNS, especially meninges, causes meningitis, or meningoencephalitis.
Humoral immunity is characterized by the appearance of antibody against the soluble S antigen and hemagglutinating antibodies. The antibody against S antigen is the first to appear, within 3-7 days after the onset of symptoms. The hemagglutinating antibodies directed against hemagglutinin confer lifelong immunity against mumps virus. CMI is essential for control of infection. This also contributes to pathogenesis of the disease and is responsible partially for the symptoms observed during the course of clinical illness. Immunity in mumps is lifelong.
The incubation period is long and varies from 12 to 25 days. Most of the infections are asymptomatic. The onset of mumps is sudden.
Complications of mumps: Meningoencephalitis is the most frequent complication of mumps in childhood. Other rare complications include oophoritis, mastitis, pancreatitis, thyroiditis, arteritis, thrombocytopenia, and pneumonia. Death due to mumps is rare.
Epidemiology
Mumps is a highly communicable disease, occurring worldwide. Mumps continues to remain endemic in many countries throughout the world, as the mumps vaccine is used in only 57% of the countries. In the absence of vaccination program, it often occurs as epidemics in children 5-15 years of age.
Humans are the only natural hosts of the mumps virus. No animal hosts are present. The infected patients are the source of infection. A patient remains infectious usually from 9 days prior to the onset of parotid swelling as long as 7 days after onset of the swelling. The infection is transmitted by direct person-to-person contact and also by inhalation of respiratory droplets.
Laboratory Diagnosis
Diagnosis of mumps is usually clinical. The laboratory diagnosis is useful for diagnosis of atypical infection or manifestation of mumps without typical symptoms.
The specimens include the saliva, urine, secretions from Stensen's duct, and the CSF.
The virus can be isolated through egg innoculation or cell culture using monkey kidney cells, human amnion cells, or HeLa cells.
Serodiagnosis The hemagglutinin inhibition, immunofluorescence assay, and ELISA are used for demonstration of viral antibodies in the serum. Detection of mumps-specific IgM antibody by IgM ELISA indicates recent and active infection.
No specific antiviral agents are available against mumps
Respiratory Syncytial Virus
Respiratory syncytial virus (RSV) is the leading cause of respiratory tract infection in infants and young children.
Morphology
It is pleomorphic and measures from 150 to 300 nm in size. It has a small nucleocapsid, measuring 13 nm in diameter, unlike large nucleocapsid (18 nm) of other paramyxoviruses. The viral envelope contains a surface glycoprotein G, by which it is attached to the cell surface of the host cells. It contains F protein but lacks both H and N proteins. The F protein induces the fusion of the infected cells with adjoining cells, resulting in the formation of large multinucleated syncytia, from which the virus derives its name.
The virus is highly labile; it is easily inactivated by dryness at room temperature and by acid. It is preserved by lyophilization.
Pathogenesis and Immunity
Respiratory syncytial virus infection is restricted to the respiratory tract. The virus initiates infection in the epithelial cells of the upper respiratory tract. Spread of the virus down the respiratory tract occurs by cell-to-cell transfer of the virus along the syncytia from the upper respiratory tract to the lower respiratory tract, resulting in pneumonia. The virus usually does not cause any viremia or systemic spread. The virus causes necrosis of the small airway epithelium, plugging of the lumens with exudates, and edema, leading to obstruction of the normal airways of the young infants.
Humoral antibody plays a minimal role in the host immunity against RSV. Maternal antibodies do not protect the infant from infection. CMI appears to play an important role in recovery from infection. Natural infection by RSV does not prevent reinfection by the virus.
Clinical Syndromes
Common cold
The illness begins with infection of the upper respiratory tract,
which manifests as common cold with marked rhinorrhea
(running nose). This condition is most common in older children
and adults. Incubation period varies from 4 to 5 days.
Bronchiolitis
In infants, RSV causes a more severe lower respiratory tract
illness, resulting in bronchiolitis or pneumonia. Clinically,
the condition presents as cough, coryza, wheezing, rales, and low-grade fever.
Epidemiology
Respiratory syncytial virus infection is prevalent worldwide. Humans are the only hosts. Infected patients are the source of infection. They continue to excrete viruses for several days or weeks in their respiratory secretions.
Laboratory Diagnosis
Specimens include respiratory secretions obtained by washing, suctioning, or swabbing of the nasopharynx.
The viral antigens in the nasal washings or nasopharyngeal aspirates can be detected by using ELISA or direct immunofluorescence antibody test using specific monoclonal antibodies. ELISA is used for demonstration of antibodies in the serum. Demonstration of a fourfold or more increase in the antibody titer of acute and convalescent sera confirms the diagnosis of RSV infection.
Treatment
Ribavirin, a broad-spectrum antiviral agent, has been recommended for the aerosolized treatment of children with severe RSV disease.
Prevention and Control
Passive immunization with anti-RSV immunoglobulin has proved beneficial for use for prophylaxis in high-risk infants such as premature babies or babies with chronic lung disease.
Attempts to use a vaccine against RSV have been proved unsuccessful to date.
Nipah Virus
Nipah virus was reported to be the causative agent of encephalitis in Malaysia and Singapore in 1998 and 1999. People rearing pig populations were increasingly susceptible to encephalitis to this previously unrecognized virus.
Hendra Virus
Hendra virus is a recently described, new paramyxovirus. Hendra virus was first isolated from the cases of severe respiratory disease in Hendra, Australia, in 1994; hence the name Hendra virus. Infection is transmitted from infected horses to humans. Fruit bats are the natural reservoir.
Human Metapneumovirus
The virus was first reported as a cause of respiratory illness in children in 2001. The clinical manifestations of HMPV are closely similar to that of RSV infection in children.
Reoviruses
The family Reoviridae is divided into nine genera, of which only four genera cause human diseases. These genera are Orbivirus, Orthoreovirus, Rotavirus, and Coltivirus.
The virus core contains many enzymes essential for transcription and capping of viral RNA. These viruses are unusually resistant to heat, a wide pH (3.0–9.0), and to lipid solvents but are sensitive to 95% ethanol, phenol, and chlorine.
Orbiviruses
Orbiviruses are primarily animal pathogens that cause disease mainly in animals. They are so named for their ring-shaped (Latin word orbi: ring) structure. These viruses are differentiated from the orthoreoviruses by their protein structure and their transmission by arthropod vectors.
They are associated with African horse sickness in horses, donkeys, and dogs; blue tongue disease in sheep; and epizootic hemorrhagic feverin deer.
The diagnostic facilities for orbivirus infections are available only in a few reference laboratories.
Coltiviruses
Coltiviruses resemble the orbiviruses in their morphology and in having two capsids. The genome consists of a 12-segmented double-stranded RNA. The coltiviruses associated with human disease include Colorado tick fever, Salmon River virus, Banna virus, Beijing virus, Gansu virus, and Eyach virus.
Colorado Tick Fever Virus
Colorado tick fever is an acute viral infection transmitted by the bite of wood tick (Dermacentor andersoni) caused by Colorado tick fever virus. Colorado fever was so named because the illness was believed to occur predominantly in Colorado and was used to distinguish this clinical illness from that of Rocky Mountain spotted fever caused by Rickettsia species. The causative agent of this fever was recognized as a virus in 1946.
The virus generally causes a nonspecific febrile illness. The incubation period is short and varies from 3 to 6 days. The clinical manifestations of the acute condition are characterized by the sudden onset of fever, chills, headache with retro-orbital pain, malaise, nausea, and occasionally vomiting. A rash is generally absent by which Colorado tick fever is differentiated from the Rocky Mountain spotted fever.
No specific antiviral treatment is available for Colorado tick fever. The condition is usually self-limited and can be prevented by avoiding contact with the wood tick.
Orthoreoviruses
Human volunteers studies have failed to establish a clear cause-and-effect relationship between reoviruses and human illness. So far, reoviruses have been linked with upper respiratory infection, fever, enteritis, and febrile exanthema in children. All three serotypes of the virus have been recovered from healthy children and from children with minor febrile illness, diarrhea, or enteritis.
No specific treatment is available for orthoreovirus infection. No preventive measures have been suggested due to the lack of definitive association of orthoreovirus with human disease.
Rotavirus
Rotavirus is the most common agent of gastroenteritis in children aged 6 months to 2 years.
Pathogenesis and Immunity
Rotavirus survives the acidic environment in the stomach and initiates infection in the mucosal cells of the small intestine. It does not cause infection in mucosa of the stomach and large intestine. After absorption, the viruses replicate in the cytoplasm of the enterocytes and damage their transport mechanism. The rotavirus after replicating in the cell causes damage to the cell. The damaged cells are released into the lumen of intestine, releasing large quantities of viruses in the diarrheic stool. It takes around 3-8 weeks for restoring the normal function of the cell. Hence, the rotavirus produces watery diarrhea similar to that seen in cholera.
Rotavirus infection is characterized by the presence of high quantity of immunoglobulin A (IgA) in the intestinal secretions. The IgA plays an important role in conferring the gut immunity against rotavirus. It protects newborns up to the age of 6 months.
Epidemiology
Rotavirus is found worldwide. The virus is an important cause of diarrhea in infants and young children between 3 and 5 years of age. The viruses are excreted in the diarrheic stool of the children 2-5 days after the start of diarrhea. The infected children are the common source and reservoir of infection. The virus is transmitted from person-to-person by fecal–oral route.
Laboratory Diagnosis
Specimen: Diarrheic stool is the specimen of choice for demonstration of rotavirus and viral antigens.
Rotavirus can be demonstrated in stool by direct electron microscopy (EM) and by immunoelectron microscopy (IEM).
Enzyme immunoassay, such as Rotazyme and latex agglutination test, are useful tools to detect rotavirus antigen directly in the stool for diagnosis of diarrheal illness. This is a method used for routine diagnosis of rotavirus diarrhea.
Treatment
No specific antiviral therapy is available for rotavirus infection. The treatment of the condition is mostly supportive. It consists of restoring the fluid loss in dehydrated patients.
Prevention and Control
Few vaccines have been evaluated to protect children from rotavirus diarrhea. Improved personal hygiene including hand washing and isolation of known cases of rotavirus are the best modes of control of the rotavirus diarrhea.
Rhabdoviruses
Rabies virus is the most important member of the rhabdoviridae family, which causes disease in humans. It causes rabies, a recognized zoonotic disease worldwide. Rabies is the most fatal infection in humans.
The family Rhabdoviridae is classified into two genera: Lyssavirus and Vesiculovirus. The genus Lyssavirus consists of more than 80 viruses and includes a rabies serogroup, which consists of 10 viruses including the classic rabies virus.
Rabies Virus
Rabies virus causes rabies, a viral infection of the central and peripheral nervous systems that causes encephalitis with or without paralysis. It is mostly fatal.
Morphology
It is a bullet-shaped virus with one end rounded or conical and the other end planar or concave. It is a negative-sense, nonsegmented, single-stranded RNA virus measuring approximately 60 X 180 nm. It is composed of an internal protein core or nucleocapsid, which contains the nucleic acid. It also consists of an outer envelope, a lipid-containing bilayer covered with transmembrane glycoprotein spikes. The nucleocapsid shows helical symmetry, containing a linear negative-sense RNA with an RNA-dependent RNA transcriptase. The virus genome is unsegmented.
Antigenic and genomic properties
G protein: The glycoprotein or G protein is present on the surface spikes present on the outer lipoprotein envelope of the virion. It mediates the attachment of virus to the acetylcholine receptors of neural tissues. The G protein is important in pathogenesis and virulence of the virus. It is strongly antigenic and elicits the production of neutralizing antibodies, which are protective.
N protein: Nucleoprotein or N protein is a group-specific antigen. It shows cross-reaction with some rabies-related viruses. It is antigenic and produces antibodies, which are not protective but are of diagnostic value.
Other antigens include membrane proteins, glycolipid, and RNA-dependent RNA polymerase.
Pathogenesis and Immunity
Rabies virus has a broad host range. The virus can infect all mammals, although certain mammals (such as dogs, foxes, wolves, and bats) are important for transmission of infection.
The virus may enter the peripheral nervous system directly at the site of bite. In some cases, however, it may replicate in muscle tissue after entering the host, remaining at or near the site of introduction for most of the incubation period.
The virus infects the sensory neurons and moves rapidly by axonal transport centripetally to the central nervous system (CNS) for replication. During its transport within the neurons, it is protected from the host immune system.
During the course of infection, encephalitis develops, associated with the death of neurons and demyelination.
Clinical Syndrome
Rabies virus causes rabies, the most fatal infection in humans. No specific antirabies agents are useful, once clinical signs or symptoms develop.
In general, four stages of rabies are recognized in humans. These are (i) incubation, (ii) prodromal period, (iii) acute neurologic period, and (iv) coma; which subsequently lead to death.
Incubation period: The average period of incubation is 20-90 days. Rarely, incubation lasts as long as 19 years. In more than 90% of cases, incubation is less than 1 year. During the incubation period, the virus travels from peripheral areas to the CNS. The patients remain asymptomatic during the period. The incubation period is less than 50 days if the patient is bitten on the head or neck or if a heavy inoculum is transferred through multiple bites, deep wounds, or large wounds. A person with a scratch on the hand may take longer to develop symptoms of rabies than a person who receives a bite on the head. The rabies virus is protected from the immune system during this period and no antibody response is observed.
Prodromal period: The virus enters the CNS during the prodromal period. The duration of this period is 2-10 days. The period is characterized by nonspecific symptoms and sign Paresthesia or pain develops at the inoculation site and is pathognomonic for rabies.
Acute neurologic period: This period is associated with objective signs of developing CNS disease. The duration is 2-7 days.
Furious rabies: Patients develop agitation, hyperactivity, restlessness, thrashing, biting, confusion, or hallucinations. After several hours to days, this becomes episodic and interspersed with calm, cooperative, lucid periods. Furious episodes last for less than 5 minutes. Episodes may be triggered by visual, auditory, or tactile stimuli, or they may be spontaneous. Seizures may occur.
Paralytic rabies: It is also known as dumb rabies or apathetic rabies, because the patient is relatively quiet compared to a person with the furious form. Paralysis develops from the outset. Fever, headache, and nuchal rigidity are prominent. Paralysis is symmetric and may be either generalized or ascending and may be mistaken for Guillain-Barre syndrome. Calmness and clarity gradually deteriorates to delirium, stupor, and then coma.
Coma: The patient may go into coma within 10 days of onset; but duration is variable. Coma leads to respiratory failure within a week of neurologic symptoms. Hypoventilation and metabolic acidosis predominate. Acute respiratory distress syndrome is common. Without intensive supportive care, respiratory depression, arrest, and death occur shortly after coma.
Most cases result in death within 14 days because of complications, despite intensive supportive care. Recovery is very unlikely. A few reports indicate that those patients who survived had pre-exposure or postexposure prophylaxis supported by most advanced life-support system.
Epidemiology
Rabies is a recognized zoonotic disease worldwide. Rabies has been recognized for over 4000 years. Today it is found in most countries, except many Australian islands, Great Britain, Japan, Hawaii, and most of the Caribbean islands.
The virus is usually transmitted from a rabid animal to humans mostly by bites or other forms of traumatic contacts. Rabies is endemic in India. It has been estimated that more than 30,000 people die of rabies in India every year. More than 70,000 people in India receive antirabies vaccination per year. With the dog population of over 16 million, the problem of rabies is significant.
Rabies is a zoonotic disease. Dogs are the important reservoir of infection. Other animal reservoirs include silver-haired bats, eastern pipistrella, raccoons, skunks, foxes, or cats, ferrets, cattle, opossums, fowl, etc. Foxes are more infectious than dogs and other animals, because larger amounts of virus are present in their saliva.
The virus is excreted in saliva of infected dogs, foxes, wolves, jackals, vampire bats, raccoons, and skunks. The virus is found in the salivary gland of these infected animals. Infected saliva or infected CNS tissue, including corneal transplants in humans, are the sources of infection.
Laboratory Diagnosis in Humans
Specimens: Saliva, serum, cerebrospinal fluid (CSF), blood, urine, and skin and brain biopsy are the frequently used specimens for diagnosis of rabies.
Microscopy: Demonstration of Negri bodies by microscopy is the characteristic histopathological feature of rabies.
Serodiagnosis: Rabies antibodies are found in the serum as well as in the CSF of human cases. A high titer of antibodies is found in the CSF. Antibodies in the serum are demonstrated by a rapid fluorescent focus inhibition test titer in which results are positive in 50% of cases.
Laboratory Diagnosis in Animals
The brain of the dead animal is the specimen of choice. The brain is collected carefully from the dead animal. Part of the specimen is collected in 50% glycerol saline (preservative) for isolation of virus. The other part of the brain including the hippocampus and cerebellum (abundant in Negri bodies) is collected in Zenkers' fixative for demonstration of Negri bodies.
Microscopy: Impression smears of the brain tissue are stained by Seller's technique for demonstration of Negri bodies. This is still a useful method in the laboratories lacking facilities for cell culture and antigen detection methods.
Treatment
No specific antirabies agent is available. Although until recently rabies was considered to be invariably fatal, it has now been demonstrated that complete recovery can occur from established rabies with intensive supportive care and management of complications.
Prevention and Control
Specific prophylaxis in rabies, depending upon whether given before or after exposure can be discussed as pre-exposure prophylaxis and postexposure prophylaxis.
Pre-exposure prophylaxis is given to persons at high risk, such as dog handlers, other animal handlers, and veterinarians. This is achieved by use of cell culture vaccines, which are more safe.
Postexposure prophylaxis is started immediately after exposure to infection. After exposure to possibly infected dog or other rabid animals, immediate preventive actions are taken up, which consist of (a) local treatment, (b) confirmation whether or not the animal is rabid, (c) administration of hyperimmune serum, and (d) antirabies vaccine.
Local treatment: This involves prompt cleaning of the wound. Animal bites deposit the virus in the wounds. The wound should be immediately scrubbed with soap and water followed by treatment with quaternary ammonium compounds, such as Cetavlon, tincture, or aqueous solution of iodine or alcohol.
Administration of hyperimmune serum: Passive immunization is carried out by administering purified equine rabies immune globulin (ERIG) and human rabies immune globulin (HRIG). Administration of HRIG is promptly made to ensure passive immunization against rabies.
Antirabies vaccine: Rabies is the only disease where postexposure vaccination is employed extensively and successfully. This is due to long incubation period of the disease.
Rabies-Related Viruses
The genus Lyssavirus consists of more than 80 viruses including the rabies virus, which is the prototypical human Lyssavirus pathogen. Other viruses included in this group rarely cause human disease.
- Duvenhage Virus: The virus has been reported in bats from Southern Africa and many European countries. The first human case was reported in 1971 from South Africa, who died of clinical rabies after being bitten by a bat.
- Mokola Virus: The virus is classified as Lyssavirus serotype 3. The virus has been isolated from many domestic and wild animals (shrew, cat, and dog) in Africa.
- Lagos Bat Virus: The virus is classified as Lyssavirus serotype 2. The viruses have been reported from bats and cats in Nigeria and Central Africa. The virus causes a rabies-like illness following intracerebral inoculation in infected monkeys.
Arboviruses
Arboviruses are RNA viruses that are transmitted by arthropods. The word arbovirus is an acronym for arthropod-borne viruses that are transmitted by arthropods, from one vertebrate host to another.
Dengue, Japanese encephalitis ( JE), yellow fever, Western equine encephalitis, Eastern equine encephalitis, St. Louis encephalitis, West Nile fever, and sandfly fever are some of the major arbovirus diseases distributed worldwide.
The Viruses
Togaviruses:
Togaviruses are single-stranded RNA viruses. They are spherical, 17 nm in diameter, and have an icosahedral nucleocapsid surrounded by an envelope. They replicate in cytoplasm of the infected host cell.
The major togaviruses are chikungunya, Venezuelan, and Western equine encephalitis viruses, Eastern encephalitis viruses, Mayaro, O'nyong-nyong, Semliki Forest, and Sindbis viruses. Most of these viruses are transmitted by mosquito arthropods to humans.
Flaviviruses
Flaviviruses are similar to togaviruses in that they are also single-stranded RNA viruses and have an icosahedral nucleocapsid surrounded by an envelope. They differ from togaviruses in being small, only 40-50 nm in diameter.
Bunyaviruses
Bunyaviruses are large, spherical (80-120 nm in diameter), and have a triple-segmented and a single-stranded RNA. The members of the family Bunyaviridae are classified into four genera: Bunyavirus, Hantavirus, Nairovirus, and Phlebovirus.
Reoviruses
Reoviruses are spherical viruses, measure 16-18 nm in diameter, and are nonenveloped viruses. The genome consists of a double-stranded RNA of 10-12 segments.
Rhabdoviruses
Rhabdoviruses include the vesiculoviruses, which are transmitted by either mosquitoes or sandflies. Vesicular stomatitis virus and Chandipura viruses are the common examples.
Arboviruses cause diseases in humans, which may be one of the
following clinical syndromes:
1. Fever with or without maculopapular rash.
2. Encephalitis, often with high mortality.
3. Hemorrhagic fever, also frequently severe and fatal.
There are some arboviruses that can cause more
than one syndrome in the infected human host, e.g., dengue
virus. Recovery from the disease usually confers a lifelong
immunity.
Arbovirus infection occurs in distinct geographical distribution and vector patterns. The viruses occur in tropics as well as in temperate countries.
Control methods essentially include vector controls and vaccination. The vaccination has been effective only in yellow fever and a few other diseases but not in most of arboviral diseases.
Yellow fever virus
Yellow fever is a mosquito-borne acute febrile illness that occurs in Africa and South America. The disease is not reported in India. Yellow fever virus is the type-specific virus of the family Flaviviridae. It is a single-stranded, enveloped, RNA virus. The envelope consists of a lipid bilayer containing an envelope glycoprotein and a matrix protein. The single RNA is complexed with a capsid protein.
ellow fever is transmitted by the bite of Ades mosquito. During blood meal, the mosquito deposits the saliva containing the virus into a bite wound. The virus replicates locally and in regional lymph nodes draining the wound. Subsequently, the virus spreads by blood to the bone marrow, liver, myocardium, spleen where further replication of virus occurs. The condition is associated with hemorrhagic manifestations occurring as a result of disseminated intravascular coagulation. The condition progresses to shock and finally death due to multiple organ failure involving liver, kidney, brain, and heart.
Host immunity is characterized by the presence of viral neutralizing antibodies by the end of the first week during which the virus is rapidly cleared from the circulation. An attack of yellow fever confers a lifelong immunity.
As the name suggests, yellow fever is characterized by jaundice and fever. This is an illness characterized by an acute onset of fever followed by jaundice within 2 weeks of onset of symptoms.
Vaccination is the most widely used preventive measure against yellow fever. Other preventive measures include mosquito eradication program and personal protective measures.
Dengue virus
Dengue is the most common mosquito-borne arboviral illness caused by dengue virus. The name dengue is derived from a Swahili word ki-dinga-pepo meaning a sudden seizure by a Demon.
The earliest known documentation of symptoms of dengue-like illness was described in Chinese Encyclopedia during 265 AD. In 1780, Rauss coined the term "break-bone fever" based on description of symptoms reported by patients during Philadelphia epidemics of probably dengue fever. The possible outbreak of dengue fever epidemics were documented sporadically every 10-30 years until World War II. Subsequently, after World War II the dengue fever spread and became worldwide. The first epidemic of dengue hemorrhagic fever was described in 1963 in Manila. In 1979-1980, the first reported outbreak of dengue fever occurred simultaneously in Asia, Africa, and North America.
Pathogenesis and immunity
Humans acquire infection and become infected with the virus by the bite of Aedes mosquito vector. The leakage of plasma caused by increased capillary permeability is the major pathophysiological abnormality that occurs in dengue hemorrhagic fever and dengue shock syndrome. Bleeding, which is most important manifestation in patients with dengue hemorrhagic fever, is caused due to capillary fragility and thrombocytopenia, and it manifests by various ways ranging from petechial skin hemorrhages to life-threatening gastrointestinal bleeding.
Infection with dengue virus confers lifelong immunity. The immunity is serotype specific. Infection by one serotype does not confer protection against other serotypes. The infection with dengue virus of different serotypes may cause a more severe disease, such as dengue hemorrhagic fever. Although dengue and yellow fever viruses are antigenically related, infection with dengue virus does not result in significant cross-immunity against yellow fever virus.
Epidemiology
Dengue virus is distributed worldwide. Dengue hemorrhagic fever is primarily a disease of children and a leading cause of death in Southeast Asia.
Humans are reservoirs of the infection. The human host serves as source of viral amplification. Humans are infectious to mosquitoes during viremia for 3-5 days. The infection is transmitted by bite of A. aegypti mosquitoes. After feeding, the virus shows an extrinsic incubation period of 10-14 days in the mosquito, before the mosquito becomes infectious. The mosquitoes are vectors as well as sources of viral amplification.
Unplanned urban development with population explosion and inadequate public health facility, poor vector control, and increased travel to the endemic areas have contributed for spread of dengue worldwide.
Diagnosis
Blood collected during first 3-5 days of illness is useful for isolation of virus, and serum is useful for serological tests. Isolation of the virus: Diagnosis of dengue is confirmed by isolation of virus from blood during first 3-5 days of illness. The virus can be isolated in various cell cultures. Serodiagnosis: Serodiagnosis of dengue fever is based on the demonstration of IgM immunoglobulin in a single serum sample or rise in IgG antibodies in paired serum specimens.
Tick-Borne Flaviviruses
Tick-borne encephalitis viruses include:
1. Russian spring-summer encephalitis
2. Powassan virus
Tick-borne hemorrhagic fever viruses include:
1. Kyasanur forest disease
2. Omsk hemorrhagic fever
Hepatitis Viruses
Hepatitis is a clinical syndrome caused by many pathogens including viruses. There are six medically important viruses that are called hepatitis viruses because their main site of infection is liver. These viruses are hepatitis A virus (HAV), hepatitis B virus (HBV), hepatitis C virus (HCV), hepatitis D virus (HDV), hepatitis E virus (HEV), and newly described G virus (HGV). Although these viruses infect the liver as common target organ, they however, differ greatly in their morphology, replication pattern, and course of infection. These viruses infect the liver and cause distinct clinical pathology by producing characteristic symptoms of jaundice and production and release of liver enzymes in the serum.
Hepatitis A Virus
Hepatitis A virus (HAV) is a picornavirus that is most commonly transmitted by fecal-oral route.
Morphology
The Hepatitis A virus is a typical enterovirus in the family Picornaviridae. It is a small, nonenveloped virus measuring 27 nm in diameter. It has a single-stranded positive-sense RNA genome. The naked capsid is more stable than other picornaviruses to acid and other treatment.
Hepatitis A virus is highly resistant to environmental factors. It is stable at 60oC for 1 hour, 56oC for 30 minutes, and 4oC for weeks. It is stable to acidic pH as low as pH 1.
The virus is inactivated by formalin (0.35%) at 37oC during a period of 24 hours
Pathogenesis and Immunity
Hepatitis A virus infection is transmitted by the fecal-oral route. The virus appears to replicate first in the gastrointestinal tract and then spreads to the liver. In the liver, the viruses infect and cause damage to hepatocytes. Cytotoxic T cells appear to cause damage to hepatocytes; hence once the infection is cleared, the cell damage is repaired and no chronic infection occurs.
Host immunity is mediated primarily by circulating antibodies. Acute infection is characterized by the appearance of IgM anti-HAV, which is detectable at the time of appearance of jaundice in the initial stage of infection. But the IgM antibody disappears several months after jaundice. The IgG antibody appears 1-3 weeks after appearance of IgM antibodies. IgG antibody appears to provide lifelong immunity against recurrent HAV infection.
Clinical Syndrome
Hepatitis A virus causes acute hepatitis A, the symptoms of which are similar to those caused by Hepatitis B virus (HBV) infection.
The incubation period of HAV is 15-45 days, with an average of 4 weeks. Fatigue, nausea, vomiting, fever, hepatomegaly, jaundice, anorexia, and rash are the most common signs and symptoms of the disease. The condition is also associated with passing of dark-colored urine, pale feces, and elevated serum transaminase levels. Hepatitis A virus infection is usually a self-limiting mild disease and in most cases resolves spontaneously in 2-4 weeks. HAV infection confers lifelong immunity. Chronic hepatitis or chronic carrier state does not occur with HAV infection.
Epidemiology
Infection with HAV occurs throughout the world but is more common in the developing countries of Asia, Africa, and Central and South America. High prevalence of HAV.
Humans are the reservoirs for HAV. Humans infected with HAV are the important source of infection. The virus is excreted in the feces during the first 2 weeks of infection, prior to the onset of symptoms; hence the quarantine of patients is not beneficial. Infected children and adults appear to be noninfectious after the appearance of jaundice.
Contaminated food or water is the main source of infection. Outbreaks can occur from a single contaminated source, such as uncooked vegetables, infected shellfish, and contaminated food and water.
Laboratory Diagnosis
Specimens include (a) serum for antibody detection test and (b) liver, bile, stool, and blood for HAV antigen and genomic analysis.
Serodiagnosis of HAV infection depends on the demonstration of specific antibodies in the serum. Serological tests demonstrating these anti-HAV antibodies in the serum are the most widely used to confirm the diagnosis of HAV infection. Enzyme-linked immunosorbent assay (ELISA) is the method of choice for detection of IgM and IgG antibodies in the serum.
Liver function tests are highly useful for supplementing the diagnosis of HAV infection. Hepatitis A virus infection is associated with a consistent increase in serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST).
Treatment
No antiviral therapy is available against HAV infection. Treatment of the condition is always supportive.
Prevention and Control
Prevention of HAV infection depends on: (a) vaccines, (b) prophylaxis with immune serum globulin, and (c) measures to prevent feco-oral spread of infection.
Hepatitis B Virus
Hepatitis B virus is a major cause of infectious hepatitis worldwide. It is a hepadnavirus, which shows restricted host range and limited tissue tropism. The virus usually causes chronic disease and is associated with hepatocellular carcinoma.
Hepatitis B virus shows following features: It is a small (3.2 kb), enveloped DNA virus. The genome is a small, circular, partially double-stranded DNA. It is partially double stranded, because its positive strand is incomplete. The complete negative strand possesses four genes: genes S, C, P, and X. The gene S codes for HBsAg and also for HBeAg (hepatitis B e antigen). The virion is a double-walled, spherical structure and measures 42 nm in diameter.
Hepatitis B virus is an extremely resistant virus capable of withstanding extreme temperature and humidity. It is stable when stored at 20oC for 15 years, at 80oC for 24 months, at 44oC for 7 days, at 37oC for 60 minutes, and at room temperature for 6 months. HBV, however, is sensitive to higher temperature and is killed rapidly after heating at 100oC for 1 minute and at 60oC for 10 hours. HBsAg is a stable antigen. It is stable at pH 2.4 for up to 6 hours, but is associated with loss of infectivity of the virus. HBsAg is also destroyed by treatment with 0.5% sodium hypochlorite within 3 minutes.
Pathogenesis and Immunity
Hepatitis B virus, after entering the blood, infects the hepatocytes in the liver with the expression of viral antigen on the surface of infected cells.
A chronic carrier stage with HBV infection is an important event in the pathogenesis of HBV infection. A person with chronic carrier stage has HBsAg persisting in the blood for at least 6 months.
Approximately 20% of HBsAg carriers, nearly 1% of all adult patients infected with HBV, and high percentage of neonates infected with the virus progress to develop hepatocellular carcinoma or cirrhosis. The hepatocellular carcinoma appears to be the result of persistent cellular regeneration that tends to replace the dead hepatocytes.
Hepatitis B virus natural infection induces a lifelong immunity. The immunity is primarily mediated by humoral antibodies against HBsAg. Antibodies to HBsAg are protective. These antibodies bind to surface antigens or with the virus and prevent it from interaction with receptors on the hepatocytes.
Clinical Syndromes
The clinical manifestations of HBV infection depend on (a) age of infection, (b) immune status of the host, and (c) the level of HBV.
Acute hepatitis B virus infection
Clinical manifestations of acute hepatitis B are similar to that of hepatitis A but with the difference that the symptoms tend to be more severe and life-threatening with HBV infection. The clinical disease associated with acute HBV infection may range from mild disease to a disease as severe as fulminant hepatitis occurring in less than 1% of the patients.
Chronic hepatitis B virus infection
Chronic HBV infection is one of the major complications of HBV infection. The risk of chronic infection is also higher in those infected at birth (90%) and in patients who are immunocompromised.
Cirrhosis and hepatocellular carcinoma are the long-term but rare complications of hepatitis B. Perinatal transmission or infection in children is associated with few or no symptoms, but infection has a high risk of becoming chronic.
Patients with chronic HBV infection have a very high risk of developing hepatocellular carcinoma. The cancer appears to be due to repeated episodes of chronic inflammation and cellular regeneration. The cancer that develops an average of 25-30 years after initial infection is the leading cause of cancer-related deaths in areas where HBV is endemic. Glomerulonephritis, polyarteritis nodosa, varieties of skin manifestations, cardiopulmonary manifestations, and joint and neurologic manifestations are other important complications of HBV infection.
Epidemiology
Hepatitis B virus is the leading cause of chronic hepatitis, cirrhosis, and hepatocellular carcinoma worldwide. Nearly, one-third of the world population is believed to be infected with HBV. More than 10% of people living in sub-Saharan Africa and in East Asia are infected with HBV. Individuals with chronic HBV infection are the major reservoir of HBV infections. These people with HBeAg in their serum tend to have high viral titers and thus greater infectivity.
Perinatal transmission: This is the major route of transmission of the virus worldwide. The transmission occurs from infected mother to child due to contact with mother's infected blood during the time of delivery as opposed to transplacental passage of the virus.
Hepatitis B virus is transmitted sexually more easily than Hepatitis C virus (HCV) or Hepatitis D virus (HDV). The infection is associated with vaginal intercourse, genital rectal intercourse, and nongenital intercourse. However, the HBV is not transmitted by hugging and kissing or by sharing towels, eating utensils, or food.
Laboratory Diagnosis
Serum is an important specimen because definitive diagnosis of HBV depends on serological testing for HBV infections. Diagnosis of acute infection is made by demonstration of HBsAg as well as HBeAg in the serum. Both HBsAg and HBeAg are the important serum markers of acute HBV. They indicate viral replication.
Treatment
No specific antiviral treatment is available for patients with acute HBV infection. Supportive and symptomatic care is more frequently used for treating HBV patients.
Prevention and Control
Hepatitis B infection can be prevented by the use of either vaccine or hyperimmunoglobulin or both.
Hepatitis C Virus
Hepatitis C virus is a flavivirus with an RNA genome and is the most important cause of parenteral non-A, non-B hepatitis (NANBH) worldwide
Morphology
Hepatitis C virus is the only member of the genus Hepacivirus in the family Flaviviridae of RNA-containing virus. HCV appears to be closely related to hepatitis D and dengue and yellow fever virus. It is a spherical, enveloped, 9.4 kb, single-stranded RNA virus with a diameter of 55 nm. The genome is approximately 9500 base pairs that encode 10 structural and regulatory proteins. Structural proteins include the core and two envelope proteins, namely, E1 and E2. These two envelope proteins undergo variation during infection due to hypervariable regions within their genes. The viruses are ether sensitive and acid sensitive.
Pathogenesis and Immunity
The ability of HCV to remain cell associated and prevent host cell death is the main determinant of viral pathogenicity, which causes persistent infection in the liver. Presence of closely related but heterogeneous population of virus genome is one of the important factors responsible for persistence of HCV infection in the liver.
Hepatocytes and possibly B lymphocytes are the natural targets of HCV. In most infected people, viremia persists and is associated with a variable degree of hepatic inflammation and fibrosis. Chronics hepatitis is characterized by lymphocyte infiltration either within the portal tract or in the liver lobule and portal and periportal fibrosis.
Clinical Syndromes
The incubation period of hepatitis C varies from 15 to 60 days with an average period of approximately 8 weeks.
HCV syndromes include: (a) acute HCV infection, (b) chronic HCV infection, and (c) cirrhosis and other complications induced by hepatitis.
Acute Hepatitis
Most patients with acute HCV infections are symptomatic and do not develop any jaundice. The symptoms of acute HCV infection tend to be mild and may appear similar to those of HBV infections.
Chronic HCV infection
Most patients with chronic hepatitis are asymptomatic and may have nonspecific symptoms, such as fatigue or malaise in the absence of hepatic synthesis dysfunction.
Cirrhosis and other complications induced by hepatitis C
Hepatitis C virus is now a leading cause of hepatitis and cirrhosis. An estimated 20% of patients with chronic hepatitis progress to cirrhosis. This process may take an average of 20 years after initial infection worldwide. Patients with this condition have a secondary risk of liver failure, portal hyper tension, and other complications.
Hepatocellular carcinoma is one of the most important complications in 1-5% of patients with underlying cirrhosis. This condition usually develops after 30 years in patients who are chronically infected and have cirrhosis.
Epidemiology
More than 3% of world's population is infected with HCV. Worldwide 170 million people are estimated to be infected with HCV. It is the most important cause of parenteral NANBH worldwide. The prevalence rates are reported to be as high as 22% in Egypt due to use of parenteral antischistosomal therapy.
Hepatitis C is exclusively a human disease. Patients who are infected with the virus are the important reservoir of infection.
Hepatitis C can be transmitted by following methods:
Blood transfusion is the most important route of transmission of HCV. The current risk of transfusion-derived HCV
HCV is transmitted parenterally (a) through transfusion of infected blood or blood products, (b) transplantation of organs from infected donors, and (c) also by sharing of contaminated needles among intravenous drug users. The use of intravenous drugs is most important risk factor responsible for around 50% of both acute and chronic infections.
Sexual transmission is believed to be responsible for approximately 20% of cases of hepatitis C. The presence of coexisting sexually transmitted disease, such as HIV, appears to increase the risk of transmission.
Perinatal transmission is possible and is observed in fewer than 5% of children born to HCV-infected mothers. The risk of perinatal transmission of HCV is higher in children born to mothers who are coinfected with HCV and HIV.
Hemodialysis, tattooing, body piercing, and acupuncture with unsterile equipments are other, but less frequent, means of transmission of HCV. Needle stick injury among healthcare workers who are exposed to infected blood accounts for nearly 4% of new infections.
Laboratory Diagnosis
Hepatitis C infection can be confirmed by employing serological tests to detect antibodies to HCV. Antibodies are directed against core envelope and NS3 and NS4 proteins and tend to be relatively low in titer. Acute HCV antibodies are usually demonstrated in acute infections 6-8 weeks after initial infection. Then antibodies that are produced persist throughout life in chronic infection.
ELISAs, including second- and third-generation ELISAs, are useful for screening of serum for anti-HCV antibodies. These assays are highly specific but cannot differentiate acute infection from chronic infection.
Treatment
A combination therapy of pegylated interferon and antiviral agent ribavirin is the current option of treatment for patients with chronic HCV infections. Other therapeutic options include the use of protease inhibitors, ribozymes, and viral vaccines.
revention and Control
No vaccine against HCV is available. Immunoglobulin is not useful in preventing transmission and, in fact, administration of immunoglobulin has been associated with HCV.
Hepatitis D Virus
Hepatitis D virus is the smallest of known human pathogens that causes infections in humans. It is an RNA virus, which is structurally unrelated to hepatitis A, B, or C virus. Hepatitis D virus is unique in being an incomplete virus and requires the presence of HBV to replicate and infect other hepatocytes.
Hepatitis D virus is a spherical, enveloped virus measuring 85 nm in diameter. It contains a single-stranded negative-sense 1.7 kb RNA. In blood, HDV or delta agent contains delta Ag (HDAg) surrounded by HBsAg envelope. HBsAg is required for HDV replication, but it may be suppressed to undetectable levels with active HDV replication.
Hepatitis D virus causes a more rapid and severe disease with rapid progression in HBV carriers superinfected with delta and HBV. Delta agent replicates in the liver, causing liver damage and cytotoxicity.
Death of hepatocytes in the liver may occur as a result of direct cytotoxic effect of HDV or through a host-mediated immune response. Hepatitis D virus is distributed worldwide. It is believed to infect approximately 15 million (5%) of world's 300 million HBsAg carriers.
The incubation period varies from 21 to 45 days but may be shorter in cases of superinfection. The clinical course of disease caused by HDV is varied and ranges from acute self-limited infection to acute fulminant liver failure. Clinically, HDV infection is indistinguishable from other forms of viral hepatitis.
No specific therapy is available for treatment of HDV infection of liver. Lamivudine and ribavirin appear to be ineffective against HBV and HDV coinfection. Antiviral therapy with interferon is also ineffective in patients with chronic infections. Vaccination with HBV vaccine protects against subsequent HDV infection. HDV virus is prevented best in the patients already infected with HBV by avoiding the use of HDV-contaminated blood or blood products.
Hepatitis E Virus
Hepatitis E virus (HEV) is the primary cause of enterically transmitted non-A non-B hepatitis virus (NANBH), most commonly seen in developing countries including India. The virus has many similarities with HAV. The virus was first observed during the electron microscopy of feces contaminated with enteric NANBH.
It is icosahedral, nonenveloped, spherical virus measuring 32-34 nm in diameter. The surface of the virion shows indentations and spikes. The virus is heat stable.
Hepatitis E virus usually causes an acute, self-limiting disease similar to HAV. Earlier it was mistaken for HAV due to clinical and epidemiological similarity. Hepatitis E virus infection, now, has been recognized as a distinct clinical entity, different from the infection caused by HAV. Clinically, HEV infection differs from HAV infection by causing acute disease and by the occurrence of symptoms much later than those of HAV disease.
Hepatitis E virus is distributed worldwide. It is most commonly found in developing countries. The epidemics of HEV have been recorded in India, Pakistan, Nepal, China, Burma, North Africa, and Mexico. During 1986-1988, one such large outbreak was reported in north-east China affecting nearly 10,000 people.
Hepatitis E virus is transmitted primarily by fecal-oral route due to fecal contamination of water in endemic areas. Fecal contaminated water is the important source of infection. The reservoir of HEV is unknown, but it may be transmitted by animals.
Treatment of HEV infection is mainly supportive. No vaccine is available now for prevention of HEV. Administration of immunoglobulin does not prevent development of clinical disease.
Hepatitis G Virus
Hepatitis G virus (HGV) is similar to viruses of Flaviviridae family, which includes HCV.
Hepatitis G virus is an RNA virus and its genome codes for 2900 amino acids. The virus shows 95% homology at the amino acid level with GB virus and GBVC, a previously described virus. HGV has 20% homology with HCV. Hepatitis G virus is a blood-borne virus, which is transmitted by transfusion of contaminated blood or blood products. HGV coinfection is observed in 6% of chronic HBV infection and in 10% of chronic HCV infection. Therefore, whether or not HGV is actually a pathogen in humans, still remains to be clarified.
Retroviruses
Retroviruses are enveloped, positive-stranded, spherical RNA viruses showing a characteristic morphology and unusual mode of replication. The presence of an unusual enzyme - the RNA-dependent DNA polymerase or reverse transcriptase, giving the virus its name (retro meaning reverse).
The enzyme reverse transcriptase prepares a DNA copy of the retroviral RNA genome-initially RNA-DNA hybrid and subsequently forms double-stranded DNA. The DNA copy of the viral genome is known as provirus. The provirus is then integrated into the host cell DNA to become a cellular gene for the rest of the life of the cell. This is in contrast to the classical transcription of the genetic information from DNA to RNA and then to proteins.
Classification
All oncogenic RNA viruses are classified in the family Retroviridae; but all retroviruses are not oncogenic.
- Oncovirinae: The Oncovirinae or oncoviruses include only the retroviruses that can transform target cells. These include human T-lymphotropic viruses (HTLV-1, HTLV-2, HTLV-3, and HTLV-4).
- Lentivirinae: The lentivirinae or lentiviruses are slow (lent: slow) viruses associated with neurological and immunosuppressive diseases in humans as well as in animals. These include human immunodeficiency viruses (HIV-1, HIV-2), Visna virus of sheep, and caprine arthritis/encephalitis virus of goat.
- Spumavirinae: These consist of spumaviruses, which contain nononcogenic “foamy viruses” (spuma: foam). These viruses are associated with asymptomatic infection in animals but are not associated with any human disease.
Morphology
The retroviruses are enveloped, mostly spherical viruses that measure 80-120 nm in size. The envelope of the virus is acquired by budding from the plasma membrane and contains viral glycoprotein.
The genome of the simple retroviruses consists of three major genes that encode glycoproteins of the virus. These are gag, pol, and env genes. The gag gene encodes for group- specific antigens, capsid proteins; pol gene for enzymes polymerase, protease, and integrase; and env gene for envelope glycoproteins. Proteolytic cleavage of the polyprotein encoded by the env gene leads to production of the viral glycoproteins.
The complex retroviruses, HTLV and the lentiviruses including HIV consist of several accessory genes, such as tat, rev, nef, vif, vpu for HIV. These genes also encode several regulatory proteins that require more complex transcriptional processing than the simple retroviruses.
Retroviruses are heat labile, readily destroyed on exposure to heat at 56oC for 30 minutes. The viruses are also readily inactivated on treatment with mild acids, ether, and formalin. They are stable on storage at 30oC.
Pathogenesis and Immunity
Provirus and oncogenes are two key components that play very important role in the pathogenesis of tumors induced by viruses. Viral oncogenes are the genes that encode proteins that induce transformation of normal cells into malignant cells. The viral oncogenes are usually of host cell origin. These oncogenes encode proteins that promote cell growth. Viral oncogenes initiate inappropriate cell growth and malignant transformation, but are not required for the replication of viruses.
Retroviruses (such as HTLV or HIV) carry a fourth gene, namely, tax or tat, next to the env gene. The tax or tat is a transactivating gene that regulates the function of the viral genes.
Clinical Syndromes
In Animals
Sarcoma and acute leukemia viruses: The sarcoma and acute
leukemia viruses cause infection primarily in animals. They are
not associated with any human infections. The avian leukosis
complex virus includes a group of antigenically related viruses,
which induce avian leukosis or sarcoma in fowls.
Leukemia viruses: These are slow oncogenic viruses, which
induce malignancies after a long latency period of even 30
years. These viruses promote cancerous growth by indirect ways
as compared to the oncogene-encoded acute leukemia or sarcoma viruses.
Epidemiology
Retroviruses are documented from different host species worldwide. Retroviruses show host specificity, the specificity being determined mainly by the presence of viral receptors on the host cell surfaces.
Laboratory Diagnosis
Most of the endogenous retroviral infection can be detected by molecular techniques, such as nucleic acid hybridization or by activation after exposure to radiation or chemicals.
Treatment
No specific treatment is available for the management of retroviral infections
Human T-Lymphotropic Viruses
HTLV-1 is one of the six distinct retroviruses known to infect human lymphocytes, others being HTLV-2, HTLV-3, HTLV-4, HIV-1, and HIV-2. HTLV and HIV are two important members of the retrovirus family.
Morphology
It is an enveloped, spherical virus measuring 100 nm in diameter. The virus consists of a single-stranded RNA with diploid genome, which has the property to replicate through DNA intermediary and is able to integrate into host T cell genome as a provirus.
HTLV is morphologically similar to HIV except that it has a centrally located nucleocapsid core in a mature virion. HTLV also differs from HIV by having a unique genome. The genome has the same gag-pol-env motif with LTR sequences as those of HIV, but it differs from HIV by having a fourth sequence (xp), which participates in transcription.
Pathogenesis and Immunity
HTLV-1 is a lymphotropic virus. The HTLVs are distinguished from HIV as they cause lymphoproliferative disorder, whereas HIV causes lymphocytosis. After entry in humans, the virus spreads through circulation and infects the CD4 helper and DTH (delayed-type hypersensitivity) T cells. These T cells are most commonly present in the skin as well as in the neurons.
HTLV predominantly infects and integrates lymphocytes. Once the infection is transmitted, the tax gene (which encodes Tax protein) transactivates the cellular genes for T-cell growth factor and IL-2 and their receptors, all of which activate growth in the infected cells. The viruses in the infected cell may remain latent or may replicate slowly for many years and may also induce the clonal outgrowth of particular T-cell clones.
The immune response to HTLV infection in humans is characteristic and depends on whether the patient develops malignancies or neuropathy. HTLV infection is characterized by the development of circulating antibodies within 4-8 weeks, which remain positive for the rest of the life. Paradoxically, the patients who have got high level of antibodies appear to be at a greater risk for HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP). These antibodies do not confer any protective immunity in patients. Cell-mediated immunity (CMI) fails to eradicate HTLV infection possibly due to ability of the virus to spread without replication, through cell-to-cell contact.
HTLV-1 is the causative agent of (a) adult T-cell leukemia (ATL), (b) HTLV-1-associated myelopathy/tropical spastic paraparesis, (c) HTLV-associated uveitis, and (d) HTLV-1-associated infective dermatitis.
Adult T-cell leukemia (ATL) is seen in approximately 1 in 20 persons infected by HTLV-1 over a period of 30-50 years. The condition is a malignancy of the CD4 helper T cells, which can be acute or chronic. Acute ATL comprises 55-75% of all the ATL cases. Lymphadenopathy (both in the periphery and within the body cavities) represents the classic form of ATL. Hepatosplenomegaly, hypercalcemia, and lytic bone lesions are the other manifestations. Death is caused due to opportunistic infections, pulmonary complications, and sepsis.
HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) is a slowly progressing degenerative disease that primarily affects the corticospinal tract of the thoracic spinal cord. The incubation period of HAM/TSP can be as short as 3 months when infection is transmitted by blood transfusion, but is usually 3 years.
Treatment
No specific treatment is available for the management of HTLV-1 infection. But a limited success has been obtained in chemotherapy of some patients with combination of AZT and interferon-alpha.
Human Immudofeciency Virus
Human immunodeficiency virus (HIV) is a retrovirus that causes acquired immunodeficiency syndrome (AIDS). AIDS is one of the most devastating epidemics ever recorded in the world. AIDS was first recognized in Los Angeles in 1981, when five cases of Pneumocystis carinii (now called Pneumocystis jirovecii) pneumonia in homosexual men and drug addicts were reported.
History
The causative agent of AIDS was first reported by Luc Montagnier and colleagues from the Pasteur Institute, Paris, in 1983. They isolated a retrovirus from a West Asian patient with persistent generalized lymphadenopathy and named it Lymphadenopathy-associated virus (LAV). In 1984, Robert Gallo and colleagues from the National Institute of Health, USA, reported isolation of a retrovirus from patient with AIDS and called it human T cell lymphotropic virus-III (HTLV-III). The International Committee on Virus Nomenclature in 1986 gave the name human immunodeficiency virus, or HIV, for the same virus. HIV-1 is first isolated virus from the cases of AIDS, and HIV-2 has been isolated from some case of AIDS from West Africa.
HIV Virus
HIV is a Lentivirus, a sub family of Lentiviridae in the family retrovirus. HIV, like other retroviruses, are enveloped RNA viruses, characteristically possessing an RNA-dependent DNA polymerase called reverse transcriptase.
Morphology
HIV genome is most complex of human retroviruses. The genome is diploid and consists of two identical copies of single-stranded positive-sense RNA genome. It contains three major genes gag, pol, and env, characteristic of all retroviruses. All these genes encode for the structural proteins.
The viral genome is surrounded by a nucleocapsid consisting of proteins. Three enzymes: (i) reverse transcriptase, (ii) integrase, and (iii) protease are located in the nucleocapsid.
The virus is surrounded by a lipoprotein envelope. The lipid component is derived from the host cell membrane and glycoproteins, which are virus coded. The major virus coded envelope glycoproteins are the projecting spikes on the surface and the anchoring transmembrane pedicles.
HIV is a thermolabile virus. It is readily inactivated at 60oC in 10 minutes and at 100oC in seconds. The virus in dried blood, at room temperature (20-25oC), may survive for up to 7 days. The virus has been isolated from various tissues even up to 16 days at autopsy of the patient infected with HIV. It is inactivated by treatment with 50% ethanol, 35% isopropanol, 0.5% lysol, 0.5% formaldehyde, 0.3% hydrogen peroxide, and 10% bleaching powder in 10 minutes. In liquid plasma or in lyophilized blood products, HIV can be inactivated by heating at 56oC for 30 minutes. It is also inactivated at a very low pH (1) and high pH (13).
Virus Isolation
Primary isolates of HIV grow very slowly on cell lines compared with laboratory-adopted strains. Virus growth is detected by testing the culture supernatant fluid to demonstrate p24 antigen or viral reverse transcriptase activity after incubation of the culture for an average period of 7-14 days or even larger (28 days).
Pathogenesis and Immunity
HIV is primarily a sexually transmitted pathogen transmitted by high-risk behaviors, such as unprotected intercourse, male homosexual intercourse, and also by intravenous (IV) drug abuse. The tropism of the HIV for CD4-expressing T-cells and macrophages is the principal determinant of the pathogenicity of HIV. HIV shows tropism for all the cells expressing CD4 antigens on their cell surfaces. This makes the patient most susceptible to opportunistic infections and certain cancerous conditions, such as Kaposi's sarcoma and lymphoma. However, the virus does not directly cause any tumor, because HIV genes are not found in these tumor cells.
In the genital tract, infection with HIV begins in Langerhans cells, the dendritic cells that line the mucosa. This is followed by infection of the local CD4 helper T cells in the genital tract and by the appearance of the virus in the blood 4-11 days after infection. The virus replicates continuously in the lymph nodes, thereby releasing the virions and infected T cells into the blood. The virus also infects brain monocytes producing multinucleated giant cells and significant central nervous system manifestations. The fusion of the HIV-infected cells in the brain and other sites is the key pathological finding
HIV is characterized by development of both cell-mediated and humoral immunities against HIV-related proteins.
Cellular immunity is characterized by the development of cellular responses produced against HIV proteins. Suppression of cell-mediated T-cell immunity is the most profound consequence of HIV infection. CD4 helper T cell, monocytes, and macrophages are important components of CMI against HIV infection. The deficiency or reduction of CD4 T cells leads to depression of cellular immune response and impairment of humoral responses.
The reduction of CD4 T cells is responsible for producing delayed-type hypersensitivity reaction that leads to opportunistic infections caused by many opportunistic pathogens. This causes CMI to gradually fail (i) to mount cytotoxic T-cell response to virally infected cells, (ii) to form delayed-type hypersensitivity reaction, and (iii) to process new foreign substances presented to the immune system.
Humoral immunity is characterized by the development of neutralizing antibodies produced against p24, gp120, gp41, and various proteins in most of the individuals infected with HIV. However, the level of neutralizing activities is low.
Clinical Syndromes
The course of untreated HIV infection is usually 10 years or longer. The disease progresses through the stages of (a) primary infection, (b) dissemination of virus to lymphoid organs, (c) clinical latency, and (d) a late stage of profound immunosuppression known as full-blown AIDS.
Acute HIV infection is characterized by rapid rise in plasma viremia with a concomitant drop in CD4 count after an incubation period of 3-6 weeks. The symptoms of HIV are nonspecific and include low-grade fever, fatigue, malaise, rash, headache, and lymphadenopathy; spontaneous resolution may occur within weeks.
The acute period is followed by an asymptomatic or clinically latent stage during which the patient continues to remain asymptomatic for several months to years.
AIDS
AIDS is the end-stage disease of the HIV infection. It denotes the irreversible breakdown of immune system of the host, making the infected host highly susceptible to a wide range of progressive opportunistic infections or unusual malignancies, such as Kaposi's sarcoma. AIDS is characterized by deterioration of immune response as evidenced by CD4 T cell decrease response.
When CD4 count falls less than 200/µL, the patient develops full-blown AIDS. This stage is characterized by development of HIV wasting syndrome with weight loss and diarrhea for 1 month. This is also associated with many opportunistic infections, such as tuberculosis, Pneumocystis carinii pneumonia, toxoplasmosis, cryptococcal meningitis, and other diseases. Patients with AIDS show clinical manifestations in different ways. They can manifest as lymphadenopathy with fever, opportunistic infections, malignancies, and neurological manifestations of HIV, such as dementia.
Opportunistic infections are the predominant causes of morbidity and mortality among the patients with late-stage HIV infection and full-blown AIDS. These are usually associated with HIV infected patients when their CD4 cell count falls to less than 200 cells/µL.
Patients with AIDS show a marked susceptibility to the development of malignancies. Human herpes virus-8-associated Kaposi's sarcoma is the most noted malignancy associated with AIDS. Kaposi's sarcoma is much more common in untreated AIDS patients than in general population. It is a vascular tumor suggested to be of endothelial origin, which is found in the skin, mucous membrane, lymph node, and visceral organs.
AIDS patients are associated with several distinct neurological syndromes. These include AIDS dementia complex, subacute encephalitis, vacuolar myelopathy, aseptic meningitis, and peripheral neuropathy. AIDS dementia complex is the most common neurological manifestation of HIV and occurs due to HIV infection of the microglial cells and neurons of the brain. This condition is characterized by poor memory, inability to concentrate, apathy, automotor retardation, and behavioral changes.
Pediatric AIDS is an important condition acquired from infected mothers. Children develop clinical manifestations by 2 years of age and subsequently die of AIDS in the following 2 years. The condition is more severe in neonates because the immune system is very poor during the time of birth. Clinical manifestations of AIDS in children include pneumonia, severe oral candidiasis, interstitial pneumonitis, encephalopathy, wasting, generalized lymphadenopathy, hepatosplenomegaly, diarrhea, growth retardation, and bacterial sepsis.
Epidemiology
HIV infection is epidemic throughout the world. HIV-1 is the most common cause of HIV infection in the Americas, Europe, Africa, and Asia. HIV is primarily a human infection. Humans infected with HIV and AIDS are the reservoir of infection. The high titer of HIV is found in the blood, semen, and vaginal secretions of the infected people; hence these are important sources of infection. The virus is also present in the breast milk of an infected mother.
Transmission of HIV infection
Sexual transmission: HIV is transmitted primarily through sexual contact and constitutes more than 70% of the HIV transmission. Sexual transmission is more common in heterosexual women and men than in homosexual men worldwide. Varied sexual behaviors, such as (a) more number of sexual partners, (b) sex with commercial sex workers, homosexuals, and (c) receptive anal sex have been reported to be increasingly associated with HIV infection. The presence of other sexually transmitted disease, such as gonorrhea, syphilis, or herpes simplex virus type 2 infection increases the risk of sexual HIV transmission by more than 100 times.
Transmission by blood transfusion: HIV is also transmitted by transfusion of infectious blood or blood products, such as serum, plasma, and cells from HIV-positive individuals.
Parenteral transmission: Parenteral transmission occurs largely among IV drug users. Injection users of illicit drugs are commonly infected through the use of contaminated needles.
Mother-to-child transmission: Mother-to-infant transmission can occur by vertical transmission or by perinatal transmission.
Laboratory Diagnosis
These include serum and plasma for HIV serology and lymphocytes for isolation of HIV.
Screening tests: Screening tests are otherwise known as ERS which is an acronym for enzyme-linked immunosorbent assay (ELISA), rapid test, and simple test. These tests are usually highly sensitive tests and are used for initial screening of the serum samples for the presence of HIV antibodies.
Virus isolation is not a routinely used method for diagnosis of HIV infection because it is time-consuming and laborious. It is used mostly for research purpose. The virus can be isolated mostly from lymphocytes in the peripheral blood and occasionally from bone marrow, plasma, and other body fluids.
Rapid tests include dot blot assay, latex agglutination, gelatin agglutination, HIV spot, and comb test, etc. These tests are simple tests, which can be performed in any laboratory without requiring any expensive instrument or skilled manpower. Moreover, test results can be read rapidly within 30 minutes of receipt of the specimen.
Treatment
Antiretroviral treatment is the mainstay in HIV treatment. The goals of antiretroviral therapy are to inhibit replication of HIV and to reduce morbidity and death.
The anti-HIV drugs can be broadly classified as: (a) nucleoside analog reverse transcriptase inhibitors (NRTIs), (b) non-nucleoside reverse transcriptase inhibitors (NNRTIs), or (c) protease inhibitors.
- Nucleoside reverse transcriptase inhibitors (NRTIs)
- Zidovudine (AZT)
- Didanosine (ddI)
- Zalcitabine (ddc)
- Lamivudine (3TC)
- Stavudine (d4T)
- Abacavir
- Non-nucleoside reverse transcriptase inhibitors (NNRTIs)
- Nevirapine (NVP)
- Delaviridine
- Efavirenz
- Protease inhibitors (PI)
- Ritonavir
- Indinavir
- Saquinavir
- Nelfinavir
- Amprenavir
- Nucleotide reverse transcriptase inhibitor
- Tenofovir
- Fusion transcriptase inhibitor
- Enfuvirtid
Prevention and Control
These include the following steps: (a) health education, ( b) screening of blood and blood products, (c) infection control, and (d) vaccine development.
Health education plays a key and important role for the prevention of AIDS in the absence of a suitable vaccine. Health education is aimed at behavioral changes including:
- Safe sexual practice by using a condom
- Not sharing unsterile needles or syringes
- Educating HIV-positive women regarding the risk of vertical transmission of HIV to infants
It is essential to screen potential blood donors before they donate blood or blood products before use. The infected persons who are tested positive for HIV should refrain from donating blood, plasma, body organs, other tissues, or sperm.
Infection control methods include the use of universal blood and body fluids precautions. These universal precautions include wearing protective clothings, such as gloves, masks, gown, etc., and using other barriers to prevent exposure to blood products. These also include disinfection of contaminated surface with 10% household bleach, 70% ethanol or isopropanol, 2% glutaraldehyde, 4% formaldehyde, or 6% hydrogen peroxide. Washing clothes in hot water with adequate detergents is effective to kill HIV.
A safe and effective vaccine is yet to be available against HIV.
Coronaviruses
Coronaviruses are a large group of viruses that cause diseases in animals and humans. They often circulate among camels, cats, and bats, and can sometimes evolve and infect people. They were named for the crown-like spike appearance of their virions on electron microscopy. These viruses are the second most important cause of the common cold; rhinoviruses being the first cause. The coronaviruses have also been reported to cause gastroenteritis in children and adults.
SARS-CoV causing atypical pneumonia called severe acute respiratory syndrome or SARS was first reported in 2002 and 2003 outbreak. This outbreak caused by the virus is believed to have originated in Guangdong province, south China. It predominantly affected mainly China, Hong Kong, Singapore, and Taiwan. Subsequently, this outbreak spread to neighboring countries in Asia, Canada, and the United States.
Morphology
Coronaviruses are enveloped viruses measuring 80-160 nm in size on electron microscopy. The glycoproteins appear as club-shaped projections (20 nm long and 5-11 nm wide) on surface of the envelope. The genome in association with N protein forms a helical nucleocapsid. The virus contains the glycoproteins E1 and E2 and a core nucleoprotein N. Some strains also contain a glycoprotein E3, which is a hemagglutinin neuraminidase.
SARS-CoV is a single-stranded, nonsegmented, plus-sense RNA virus. It measures approximately 30 kb in length. The genomic sequence of SARS-CoV is different from other coronavirus strains. These strains of SARS-CoV are quite stable unlike other coronaviruses in which mutations in the RNA sequence during replication of virus are common.
Pathogenesis and Immunity
The coronaviruses are confined strictly to the mucosal cells of the respiratory tract. These viruses typically cause infections in the upper respiratory tract, because the optimal temperature for replication of viruses is 37-38oC. The envelope contains (a) E2 viral attachment protein, (b) E1 matrix protein, and (c) N1 nucleocapsid protein.
SARS-CoV causes infection in the respiratory tract by binding to angiotensin-converting enzyme 2 receptors on the surface of respiratory epithelium. This causes alteration in fluid balance and leads to development of edema in alveolar space. Diffuse edema resulting in hypoxia is characteristic of pneumonia caused by SARS-CoV.
Clinical Syndromes
Coronaviruses cause following syndromes: (a) common cold, (b) gastroenteritis, and (c) SARS.
Common cold caused by coronaviruses has an incubation period of 3 days. The condition is characterized by rhinorrhea, sore throat, and low-grade fever. The condition typically lasts for several days.
The coronaviruses have also been reported to cause gastroenteritis in children and adults. The symptoms are mild, and the condition is self-limiting.
Severe Acute Respiratory Syndrome - SARS
SARS is a potentially life-threatening infection associated with the onset of flu-like syndrome, which may progress to pneumonia, respiratory failure, and in some cases death. The incubation period varies from 2 to 7 days, although it may be as long as 2 weeks.
Morbidity and mortality due to SARS is more in elderly population and also seen in more individuals with coexisting chronic illness and immunosuppression. The overall mortality is more than 10% and is more than 50% in elderly individuals above 65 years
Epidemiology
The SARS outbreak in 2002-2003 predominantly affected mainland China, Hong Kong, Singapore, Taiwan, and Canada. The SARS strain is believed to have originated in Guangdong province in southern China. The disease is epidemiologically linked to the National Institute of Virology in Beijing, where the outbreak is thought to have originated. A total of 8098 cases, 774 deaths, and 7324 recoveries from SARS were documented between November 2002 and April 21, 2004.
It is suggested that SARS-CoV may have originated in
chickens, ducks, or small mammals. In these animals, the
virus would have mutated, subsequently being transmitted,
thereby causing infection in humans. The transmission to
humans is facilitated possibly by the proximity in which
humans and livestock live in rural southern China. The
avian flu epidemic in Hong Kong in 1977, which originated
in poultry and spread to humans (resulting in the slaughter
of millions of chickens), is a classical example of this type of
zoonotic transmission.
It is also postulated that the SARS-CoV initially originated
in civet cats, which were sold in a Guangdong marketplace
in rural southern China as a food delicacy. Close contact
with their saliva or feces, or with the animals themselves,
possibly has transmitted a mutated SARS-CoV to humans.
Laboratory Diagnosis
Specimens include respiratory secretions for isolation of virus, and serum for testing of antibodies.
SARS-CoV can be isolated in viral cultures. Isolation of virus is attempted only in class III laboratories.
Serodiagnosis of SARS depends on detection of specific antibodies to SARS-CoV in serum obtained during acute illness or 28 days and more after the onset of disease.
Treatment
No specific antiviral treatment is available against SARS. Treatment is mostly symptomatic as given for a serious community-acquired pneumonia
Prevention and Control
Isolation of patient and strict barrier nursing is crucial to prevent transmission of SARS to others. Moreover, airport screening for potentially sick and/or febrile passengers is being carried out in SARS-affected regions in Asia by using infrared scanners. These scanners identify potentially febrile passengers by measuring their body heat. The software in the scanner is color-coded in temperature ranges; as skin temperature increases, the colors on the scanner change, such as black to green to yellow and, finally, to red. Any individual with a skin temperature of 37.5oC or greater glows bright red on the scanner. This, however, shows a lot of false-positive reactions, as many other noninfectious conditions (sunburn, ingestion of alcoholic beverages, recent cigarette smoking, or brisk exercise, etc.) can cause an increase in the skin temperature.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
SARS-CoV-2 is a strain of coronavirus that causes COVID-19 (coronavirus disease 2019), the respiratory illness responsible for the ongoing COVID-19 pandemic. It was first identified in the city of Wuhan, Hubei, China, the World Health Organization declared the outbreak a Public Health Emergency of International Concern on 30 January 2020, and a pandemic on 11 March 2020.
Human-to-human transmission of SARS-CoV-2 was confirmed on 20 January 2020 during the COVID-19 pandemic. Transmission was initially assumed to occur primarily via respiratory droplets from coughs and sneezes within a range of about 1.8 metres (6 ft). Other studies have suggested that the virus may be airborne as well, with aerosols potentially being able to transmit the virus.
An epidemiological model of the beginning of the outbreak in China suggested that "pre-symptomatic shedding may be typical among documented infections" and that subclinical infections may have been the source of a majority of infections.
Reports have indicated that reinfection can occur with variable severity. The first reported case of reinfection was a 33-year-old man from Hong Kong who first tested positive on 26 March 2020, was discharged on 15 April 2020 after two negative tests, and tested positive again on 15 August 2020 (142 days later), which was confirmed by whole-genome sequencing showing that the viral genomes between the episodes belong to different clades.
Antigenic Variants
There are many thousands of variants of SARS-CoV-2, which can be grouped into the much larger clades.
Alpha: Lineage B.1.1.7 emerged in the United Kingdom in September 2020, with evidence of increased transmissibility and virulence. Notable mutations include N501Y and P681H. An E484K mutation in some lineage B.1.1.7 virions has been noted and is also tracked by various public health agencies.
Beta: Lineage B.1.351 emerged in South Africa in May 2020, with evidence of increased transmissibility and changes to antigenicity, with some public health officials raising alarms about its impact on the efficacy of some vaccines. Notable mutations include K417N, E484K and N501Y.
Gamma: Lineage P.1 emerged in Brazil in November 2020, also with evidence of increased transmissibility and virulence, alongside changes to antigenicity. Similar concerns about vaccine efficacy have been raised. Notable mutations also include K417N, E484K and N501Y.
Delta: Lineage B.1.617.2 emerged in India in October 2020. There is also evidence of increased transmissibility and changes to antigenicity.
Omicron: Lineage B.1.1.529 emerged in Botswana in November 2021.
Treatment and drug development
Very few drugs are known to effectively inhibit SARS-CoV-2. Masitinib is a clinically safe drug and was recently found to inhibit its main protease, 3CLpro and showed >200-fold reduction in viral titers in the lungs and nose in mice. However, it is not approved for the treatment of COVID-19 in humans. In December 2021, the United States granted emergency use authorization to Nirmatrelvir/ritonavir for the treatment of the virus; the European Union, United Kingdom, and Canada followed suit with full authorization soon after.
Other Viruses
Rubella Virus
Rubella virus causes rubella, a mild viral disease affecting the skin, lymph nodes, and less commonly, the joint. It also causes congenital rubella syndrome. It is an RNA virus classified as a rubivirus in the family Togaviridae. Initially rubella virus infects the nasopharynx of the upper respiratory tract and then spreads to local lymph nodes. From there, the virus spreads by the blood throughout the body to the internal organs and skin. The occurrence of mild rash is characteristic. The exact cause of pathogenesis of rash is not known, but may be due to antigen–antibody mediated vasculitis.
Incubation period varies from 14 to 21 days. Rubella is a milder and more subtle disease than measles. A three-day maculopapular or macular rash, which starts on the face and progresses downward to involve the extremities, is the characteristic presentation in symptomatic cases. The rash typically lasts 3 days. Tender lymphadenopathy that affects all the nodes but most commonly affects suboccipital, postauricular, anterior, and posterior cervical nodes is the hallmark of rubella.
Norwalk Virus
orwalk virus is one of the most common causes of viral gastroenteritis in adults. Norwalk viruses are highly contagious. As low as 100 viral particles can establish infection, which is typically limited to the mucosal cells of the intestinal tract. Infection is characterized by damage to microvilli in the small intestine, causing malabsorption. The virus-mediated changes in gastric mucosa and delayed gastric emptying cause vomiting. It is a noninvasive virus and does not cause invasion of the colon; therefore leukocytes or erythrocytes in the feces are typically absent. No histopathological lesions are seen in the gastric mucosa.
Viral Hemorrhagic Fever
Most viral hemorrhagic fevers (VHF) are caused by 12 distinct enveloped RNA viruses that belong to four families: Arenaviridae, Bunyaviridae, Filoviridae, and Flaviviridae.
Ebola and Marburg are two most important causative agents of VHFs with a mortality of 25-100%. Both viruses are found in Africa and possibly in Philippines.
Incubation period of Ebola and Marburg infection varies from 2 to 14 days. Symptoms are nonspecific. An insidious or sudden onset of fever, chills, malaise, generalized myalgias and arthralgias, headache, anorexia, and cough are some of the common symptoms. The condition may also be associated with sore throat, epigastric pain, vomiting, and diarrhea.
Arenavirus
Arenaviruses primarily infect macrophages and cause the vascular damage. Pathogenesis of arenaviruses infection is largely attributed to T-cell immunopathogenesis. T-cell-induced immunopathogenic effect contributes to the exacerbation of tissue destruction.
Arenaviruses cause the following diseases:
- Lymphocytic choriomeningitis
- Lassa fever
- South American hemorrhagic fever.
Ribavirin is the antiviral drug used for the treatment of Lassa fever and also for South American hemorrhagic fever. The treatment is mostly supportive to maintain fluid and electrolyte balance, which can be lifesaving.
Visna
Visna is a disease of sheep caused by visna virus, a lentivirus. It is a single-stranded RNA virus with an RNA-dependent DNA polymerase in the virion. Integration of the DNA provirus into the host cell DNA appears to be responsible for persistence of the virus within the host. Clinically, the condition manifests after a long incubation period with a prolonged progressive course. It has an insidious onset with paresis, progressing to total paralysis and death. The viruses are present in the saliva, blood, and CSF of the infected animal. High levels of serum antibodies are present in the serum, but these are not protective.
Prions and Prion Diseases
Some of the diseases caused by prions include:
- Kuru
- Creutzfeldt-Jakob disease (CJD)
- Gerstmann-Straussler-Scheinker syndrome
- Fatal familial insomnia and sporadic fatal insomnia
- Scrapie
- Bovine spongiform encephalopathy
- Feline spongiform encephalopathy
- Transmissible mink encephalopathy
- Chronic wasting disease of deer, moose, and elk
In Humans-
In Animals-
Prions are small protein-containing infectious particles with no detectable nucleic acid. They were suspected to be viruses but otherwise do not conform to the standard definition of viruses. The prions show following characteristics:
- Prions like viruses are filterable.
- They apparently lack any virion structure or genome.
- Unlike viruses, they are unusually resistant to inactivation by heat, disinfectants, and radiation.
- They do not elicit any specific immune response in the infected host.
The normal prion protein known as prion protein cellular or PrPC has a significant amount of helical configuration. In its alpha-helical configuration, PrPC is usually sensitive to degradation by the activity of enzyme protease. Disease occurs when the PrPC is reconfigured into the betasheet configuration known as prion protein scrapie or PrPSC, which is resistant to degradation by protease. These abnormal proteins are resistant not only to protease degradation but also to radiation, heat, and other agents that destroy proteins. These aggregate into filaments that disrupt neuron functions and cause death of cells. Both the normal alphahelical form and the abnormal beta-pleated sheet form have the same amino acid sequence but differ in their configuration.
Pathogenesis and Immunity
Prion disease is transmitted either orally or transcutaneously. But how the prions reach the CNS, their target, is yet to be understood fully.
In experimental animal studies carried out in chimpanzees, Prusiner demonstrated that scrapie protein (PrPSC) binds to the normal cellular prion protein (PrPC) of the host. PrPC is found in most tissues of the body but is expressed in higher quantities in the CNS particularly the neurons, the main site of action of prions. PrPSC is ingested by neurons and phagocytic cells but continues to remain intact without being degraded. This may contribute to vacuolation of the neuron, a key pathological change observed in encephalitis caused by prions. In addition, accumulation of prions in high concentration causes damage in the brain tissue.
Clinical Syndromes
Kuru
Kuru is a fatal neurological disease described only among Fore tribe inhabiting the highlands of New Guinea. The disease was spread by cannibalistic funeral practice of the tribal population. This involved the ritualistic practice by the closest female relatives and children usually consuming the brain of the person following his or her death. The brain which contained most of the infectious pathogen was the source of infection. Kuru has largely disappeared today because cannibalism has been abolished now among the Fore tribal people. Kuru is a fatal disease characterized by progressive cerebellar ataxia and tremors. The condition manifests initially as difficulty in walking, followed by cerebellar tremor, hence the name kuru, which means 'trembling in fear'. Eventually the tremor worsens, followed by progressive cerebellar ataxia and eventual death within a year of onset of symptoms. The clinical course lasts for 3 months to 2 years.
Creutzfeldt-Jakob disease
CJD is the most common prion disease responsible for nearly 85% of all human prion diseases. The condition was described by Creutzfeldt (1920) and Jakob (1921), after whom the disease is named. Creutzfeldt-Jakob disease is a subacute progressive encephalopathy characterized by a rapidly progressive dementia, associated with myoclonic jerks. Memory loss, behavioral changes, and confusion are the common clinical manifestations. The condition is also associated with ataxia, aphasia, visual loss, and hemiparesis. The condition progresses and in the terminal stage of the disease, the patient becomes mute and comatose. The condition is associated with extensive cortical spongiosis, gliosis, and neural loss. The condition is invariably fatal and death occurs in about 8 months.
Variant CJD
The vCJD is believed to be caused by ingestion of BSE-infected beef products contaminated by neural tissues. This is because neural tissues have a much higher concentration of PrPSC compared with any other non-neural tissue. The condition was first documented in Britain in 1985.
Gerstmann-Straussler-Scheinker syndrome
The condition was originally described in 1936 as affecting humans. The main clinical findings of the condition are slowly progressive limb and truncal ataxia, as well as dementia. Death of the patient usually occurs within 3-8 years of presentation of symptoms.
Fatal familial insomnia
Patients with this illness present with intractable insomnia, dysautonomia, dementia, and motor paralysis. Death occurs within 6 months to 3 years following presentation.
Scrapie:
Scrapie is a prototype prion disease of sheep, known for centuries. The infection is transmitted vertically from ewe to lamb and less frequently by direct contact. Incubation period is nearly 2 years. The condition manifests as intense irritation, and to relieve that the infected animals scratch themselves, against trees and rocks, hence the name scrapie. The condition progresses to emaciation and paralysis and finally leads to death of the animal. Autopsy of the infected brain shows spongiform degeneration without inflammation in the brain tissue. The condition has been documented extensively.
Bovine spongiform encephalopathy (BSE) - Mad Cow Disease
The disease is relentlessly progressive until the animal dies. The cattle feed containing prion-contaminated meat and bone meal, which was used as a protein source, was the source of infection for trans mission of BSE to cattle. The mad cow disease, documented in 1986, has been described in cattle in the European countries and in the United States. Till 2004, nearly 190,000 confirmed clinical cases of BSE in cattle have been reported worldwide; the majority of which were from the United Kingdom alone.
Transmissible mink encephalopathy
Transmissible mink encephalopathy is a scrapie-like disease of mink. The causative agent is believed to be a strain of scrapie virus, which is transmitted to mink by feeding the animals on scrapie-infected sheep meat.
Chronic wasting disease
It is a progressive wasting disease of deer, moose and elk, initially reported in the United States but has now been diagnosed in Canada, Norway and South Korea.
Treatment, Prevention and Control
No specific treatment is available for any prion disease.
Prions are highly resistant to inactivation by disinfectants used for other viruses, such as formaldehyde, detergents, and ionizing radiations. Hence, the materials from patients with CJD or vCJD must be handled with special care. For prevention of these diseases, special disinfection protocols have been developed by the World Health Organisation (WHO). These include autoclaving at 15 lbs for 1 hour (instead of 20 minutes) or treatment with 0.1 M sodium hydroxide and 5% hypochlorite solution.
References
- Subhash Chandra Parija, 2012. Textbook of Microbiology and Immunology - 2nd Edition. Published by Elsevier, a division of Reed Elsevier India Private Limited.